Meleumycin enteric capsule and preparation method thereof
A technology of mebelamycin and enteric-coated capsules, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, and medical preparations containing active ingredients. It can solve the problems of poor dissolution rate and stability, lack of mebelamycin, The bioavailability is not high, and the effect of good reproducibility, simple process and good enteric effect is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
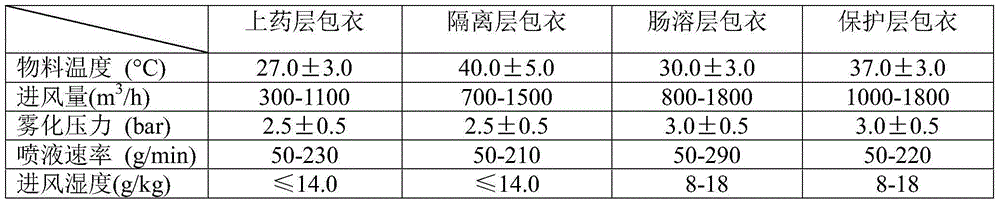
[0015] Weigh 40 parts of melemycin, 15 parts of copovidone, 6 parts of magnesium oxide, 800.5 parts of polysorbate, and 210 parts of isopropanol to prepare the coating solution for the coating layer; weigh 15 parts of copovidone, talc 32 parts of powder and 110 parts of purified water were used to prepare the coating solution for the isolation layer; 260 parts of acrylic resin, 6 parts of triethyl citrate, 3 parts of glyceryl monostearate, 800.5 parts of polysorbate, and 160 parts of purified water were weighed respectively. 3 parts of hydroxypropyl methylcellulose, 3 parts of talcum powder, and 60 parts of purified water were weighed to prepare the coating solution of the protective layer;
[0016] Add the blank pellet core into the GPCG30 multifunctional fluidized bed, and apply the above-mentioned coating solutions to the drug layer coating, isolation layer coating, enteric coating layer coating, and protective layer coating respectively. After sieving, Just dry.
[0017] ...
Embodiment 2
[0019] Weigh 45 parts of mebamycin, 16 parts of copovidone, 8 parts of magnesium oxide, 803 parts of polysorbate, and 220 parts of isopropanol to prepare the coating solution for the coating layer; weigh 16 parts of copovidone, talc 40 parts of powder and 120 parts of purified water were used to prepare the coating solution for the isolation layer; 270 parts of acrylic resin, 8 parts of triethyl citrate, 5 parts of glyceryl monostearate, 802 parts of polysorbate, and 170 parts of purified water were weighed respectively. 4 parts of hydroxypropyl methylcellulose, 5 parts of talcum powder, and 70 parts of purified water were weighed to prepare a coating solution for the protective layer; the blank ball core was added to the GPCG30 multifunctional fluidized bed Inside, each of the above-prepared coating solutions is respectively coated with a drug layer, an isolation layer, an enteric layer, and a protective layer, and then dried after sieving.
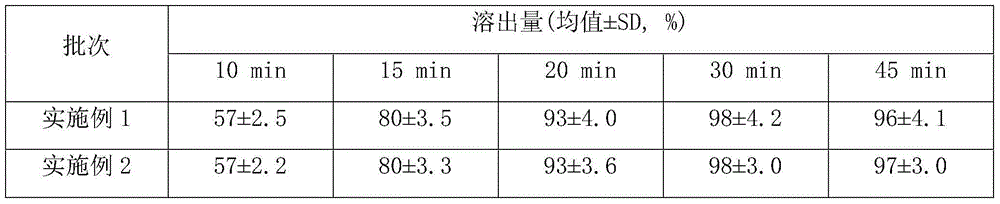
[0020] Twelve capsules were rando...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com