A kind of separation and purification method of doramectin
A doramectin, separation and purification technology, applied in chemical instruments and methods, organic chemistry, preparation of sugar derivatives, etc., can solve problems such as inability to meet drug quality requirements, unfavorable industrial production, and low product purity, and achieve production costs. Low, environmentally friendly, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
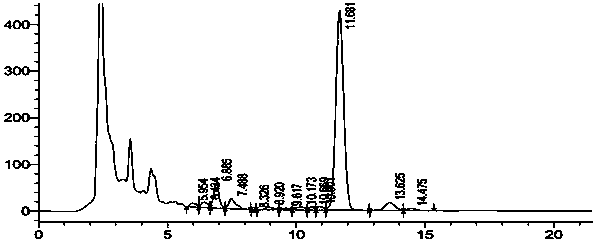
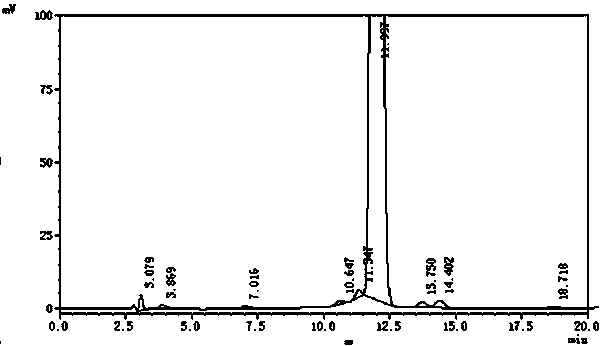
[0030] Take 20L doramectin fermentation broth (fermentation unit 1.1 g / L, HPLC spectrum as figure 1 shown), add 800g of diatomaceous earth, stir evenly, and filter with a plate and frame filter press to obtain 6.3kg of filter residue. Add 18.9L of 95% methanol, keep the temperature at 50°C, stir for 8 hours, filter with suction, wash the filter cake with 4L of 95% ethanol, and collect about 22L of filtrate. Control the temperature at 50°C, concentrate the filtrate to a paste, the weight of the extract is about 58g, dissolve the extract with 2.3L of 65% ethanol, then add 46g of activated carbon, control the temperature at 50°C, stir for 2 hours, filter with suction, and use 200mL of 65% Wash the filter cake with ethanol, collect 2.5L of filtrate, add purified water to the filtrate continuously at a speed of 75ml per minute to dilute, stir while diluting until the ethanol concentration in the solution reaches 33%, then cool down while stirring until the temperature drops to 10...
Embodiment 2
[0032]Take 20L of doramectin fermentation broth (fermentation unit: 1.1g / L), add 400g of perlite, stir evenly, filter with a plate and frame filter press, and obtain 6.1kg of filter residue. Add 12.6L of 90% ethanol, keep the temperature at 40°C, stir for 5 hours, filter with suction, wash the filter cake with 4L of 90% ethanol, and collect about 16L of filtrate. Control the temperature at 40°C and concentrate the filtrate to a paste under reduced pressure. The weight of the extract is about 46g. Dissolve the extract with 920ml of methanol with a concentration of 60%, then add 69g of activated carbon, control the temperature at 50°C, stir for 2 hours, and filter with suction. The filter cake was washed with 100mL of 60% methanol, the filtrate was collected, and purified water was continuously added to the filtrate at a rate of 10ml per minute, and stirred while diluting until the methanol concentration of the solution reached 30%, and then cooled while stirring until the temper...
Embodiment 3
[0034] Take 20L of doramectin fermentation broth (fermentation unit 1.1 g / L), add 1600g of diatomaceous earth, stir evenly, filter with a plate and frame filter press, obtain 6.7kg of filter residue, heat and dry at 50°C to obtain dry bacterial residue About 2.72 kg, add 13.6L butyl acetate, keep the temperature at 60°C, stir for 15 hours, filter with suction, wash the filter cake with 4L butyl acetate, and collect about 17.5L of filtrate. Control the temperature at 60°C and concentrate the filtrate to a paste under reduced pressure. The weight of the extract is about 42g. Dissolve the extract with 2.1L of 70% acetone, then add 25g of activated carbon, control the temperature at 50°C, stir for 2 hours, and filter with suction. Wash the cake with 100mL of 70% acetone, collect 2.2L of filtrate, add purified water continuously to the filtrate at a rate of 110ml per minute, stir while diluting until the concentration of acetone in the solution reaches 35%, then cool down while stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com