Tauroursodeoxycholic acid capsule and preparation method thereof
A technology of tauroursodeoxycholic acid and capsules, which can be used in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as large gaps and fast dissolution rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Effect of Capsule Contents on Dissolution Behavior
[0027] The composition of the pre-test sample capsule contents is
[0028]
[0029] Preparation Process:
[0030] ① Pass taurursodeoxycholic acid and other auxiliary materials through an 80-mesh sieve, and set aside.
[0031] ②Precisely weigh the prescribed amount of tauroursodeoxycholic acid, starch, lactose, microcrystalline cellulose, and magnesium stearate, first mix the excipients except the main drug evenly, and then mix the main drug with the premixed excipients uniform.
[0032] ③Put the mixed material in the capsule filling machine, and adjust the filling capacity for capsule filling.
[0033] ④ Intermediate detection, determination of dissolution curve, calculation of f2 factor.
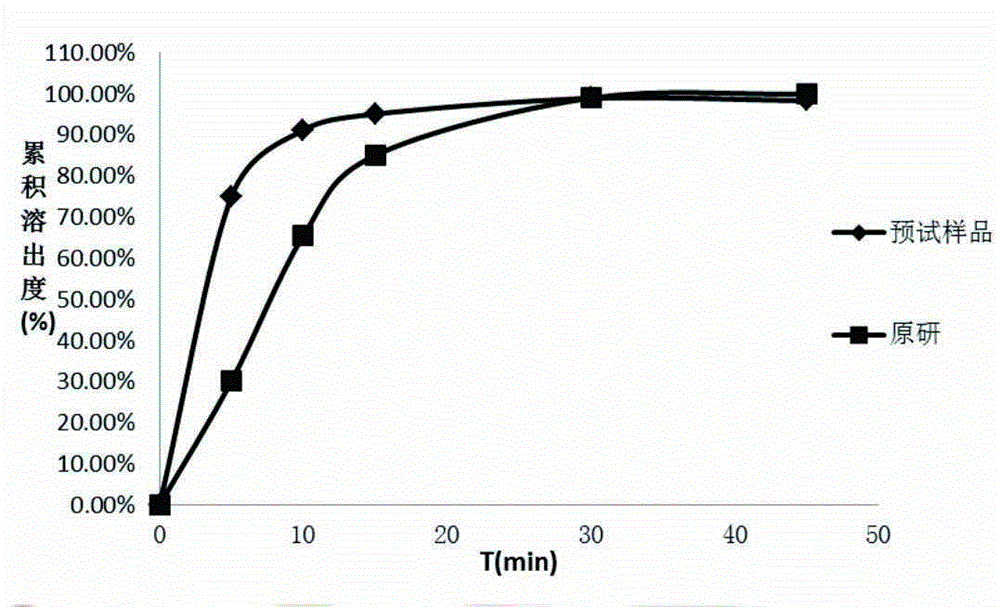
[0034] Using the above prescription process to prepare small test samples, the prepared samples are compared with commercially available products for dissolution, the results are shown in figure 1 . It can be se...
Embodiment 2
[0035] Example 2: Effect of Binder on Dissolution Behavior
[0036] Table 2: The composition of the sample capsule contents is:
[0037]
[0038] Binder 1 was ethyl cellulose and Binder 2 was 10% starch slurry.
[0039] The above prescriptions 21-25 are prepared into finished products, and the preparation process is as follows:
[0040] (1) Raw material pretreatment: Tauroursodeoxycholic acid is passed through an 80-mesh sieve or crushed and passed through an 80-mesh sieve;
[0041] (2) Recipe: receive and pass the inspection of tauroursodeoxycholic acid (calculated as dihydrate), starch, lactose, microcrystalline cellulose, binders (hypromellose, etc.), magnesium stearate, Highly substituted hydroxypropyl cellulose, weighed according to the batch prescription.
[0042] (3) Preparation of the adhesive: according to the composition of the adhesive prescription, slowly add high-substituted hydroxypropyl cellulose into 30 parts of vigorously stirred purified water, mix well...
Embodiment 3
[0053] Embodiment 3: the influence of stabilizer on dissolution behavior
[0054] Table 3 Composition of Sample Capsule Contents
[0055]
[0056] Stabilizer 1 is glyceryl behenate, and stabilizer 2 is ethyl cellulose.
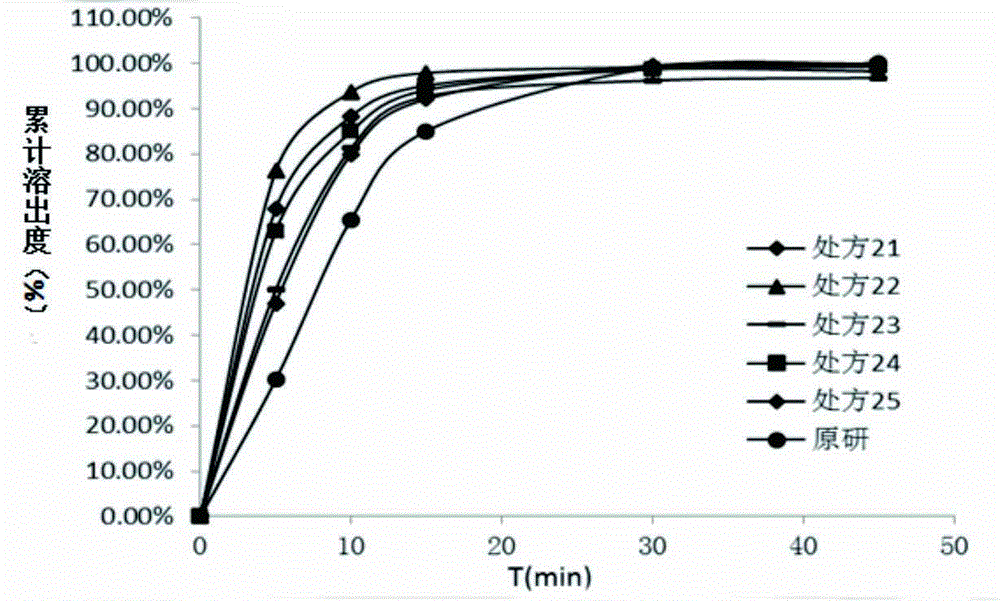
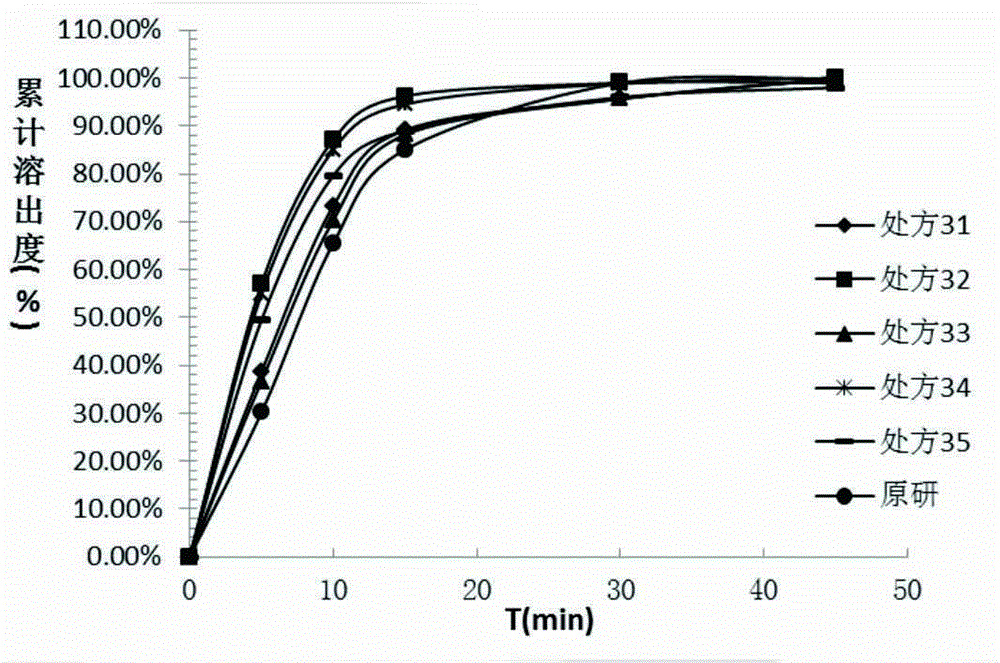
[0057] The method in the above-mentioned prescription reference example 2 was prepared into capsules, and the dissolution test was carried out under the same conditions as the original research capsules, and the second method of appendix XC of the second part of the Pharmacopoeia of the 2010 edition was used for water and pH5.5 phosphate buffer. The dissolution test, the experimental results are as follows image 3 , Figure 4 shown.
[0058] Result analysis:
[0059] A. From image 3 It can be seen that the curves of prescription 31 and prescription 33 are relatively close, indicating that small-scale changes in the amount of excipients have little impact on the dissolution behavior and do not have a significant impact.
[0060] B. Compared with the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com