Cell production method for influenza virus vaccine
A technology for influenza virus and production method, which is applied in the field of cell production of influenza virus vaccine, can solve the problems of hidden danger of vaccine safety and excessive production cycle, and achieve the effects of reducing production cost, short production cycle and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
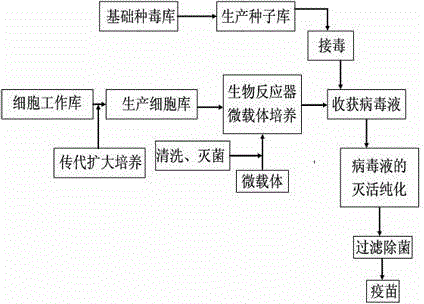
[0024] Such as figure 1 Shown, the cell production method of influenza virus vaccine is characterized in that, comprises the following steps:
[0025] 1) Preparation of seed poison
[0026] a) Passage and culture of cells: Digest and passage Vero cells with Thermo scientific HyQTase-trypsin cell dispersion, and continue to culture with HycloneSFM4MegaVir or equivalent serum-free medium cell growth medium to form monolayer cells;
[0027] b) Inoculation and propagation of seed virus: use cell maintenance solution to dilute the H1, H3 and B-type epidemic strains provided by WHO in 10 -4 Inoculate the well-growing monolayer cells above, and add 3ug / L Type IX trypsin at the same time, continue to cultivate, and harvest the virus liquid when the cells are more than 50--100% diseased; then use the virus liquid as a seed virus on the cells Carry out cell adaptive continuous passage for 6 generations as production seeds;
[0028] 2) Microcarrier suspension culture of Vero cells in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com