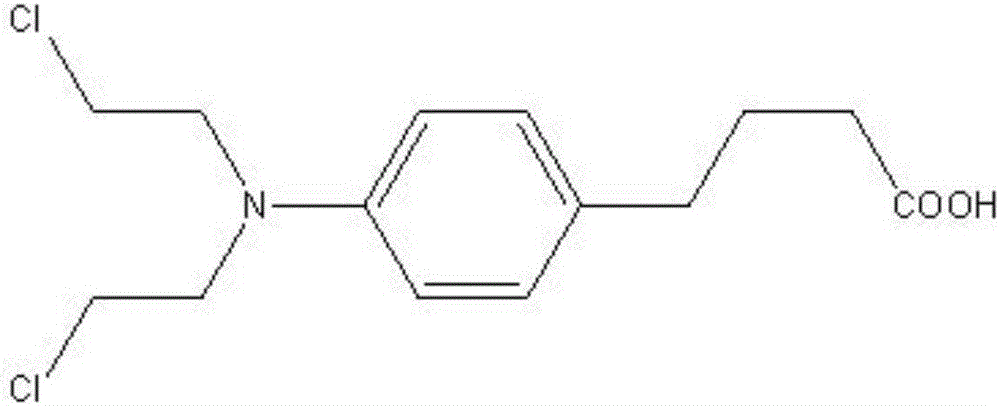
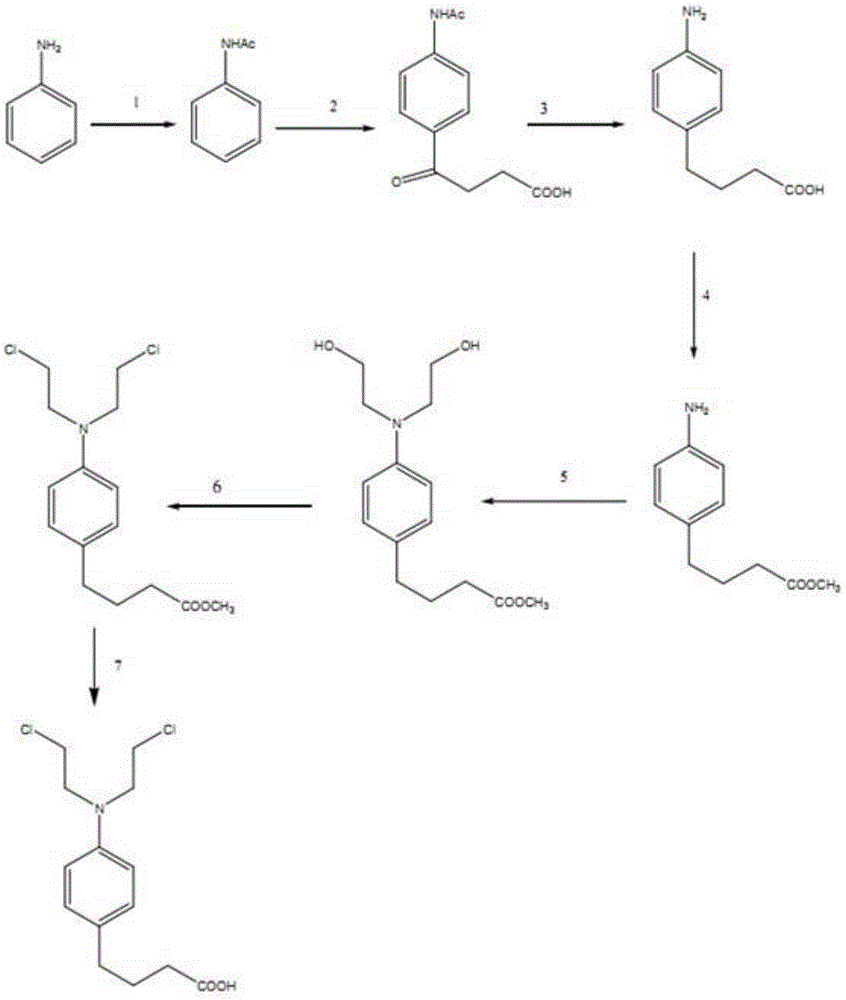
A kind of synthetic technology of antineoplastic drug chlorambucil
A chlorambucil and a synthesis process technology, applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of high toxicity, high cost, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be further described below in conjunction with specific examples.
[0028] Step (1) amino protection reaction:
[0029] Mix 93.2g of aniline and 900g of water into a 2000ml three-necked flask, stir mechanically, control the temperature below 45°C, add 103.2g of acetic anhydride, stir for 20min, filter, wash the filter cake with water, and dry it at 60°C to obtain white luster Crystalline powder 104g, the yield is 81%.
[0030] Step (2) acylation reaction:
[0031] Add 400g of aluminum chloride and 800ml of dichloromethane into a 3L three-necked flask, cool in an ice bath, mix 160g of acetanilide and 120g of succinic anhydride and add in portions, remove the ice bath after 15 minutes, react at room temperature for half an hour, and let it stand After 2-3 days, add ice water (about 1.5Kg) to generate a large amount of hydrogen chloride gas, stir, filter, wash with water, dissolve the filter cake with 4N aqueous sodium hydroxide solution, extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com