Method for the synthesizing ormetoprim
A synthetic method, the technology of sodium methoxide, applied in the direction of organic chemistry, etc., can solve the problems of unfavorable labor protection and environmental protection, unfavorable for large-scale production, not easy to obtain, etc., and achieve the synthesis process of environmental protection, low cost and short synthesis cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
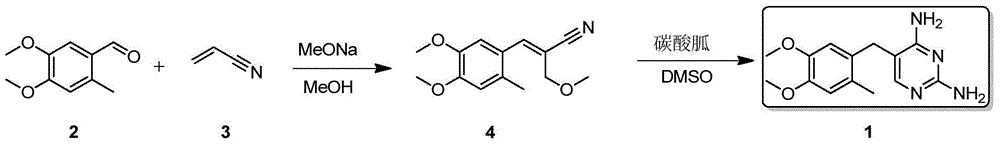
[0018] Embodiment 1, the synthesis of Omeprene
[0019] Sodium methoxide (270g, 5mol) was slowly added to methanol (900mL) solution, then 3-methoxypropionitrile (170g, 2mol) and 4,5-dimethoxy-2-methylbenzene were added to the reaction solution Formaldehyde (180g, 1mol), and react at 50-55°C for 24 hours, then add guanidine hydrochloride (191g, 2mol) in batches, and react at 50-55°C for 24 hours, cool to room temperature, filter, and wash the filter cake with a small amount of water , Obtain Omepleline (197g) after product drying, yield 72%. White solid, melting point: 232.5~233.0℃, 1 HNMR (500MHz, d 6 -DMSO):8.2(br s,2H),7.8(br s,2H),6.86(s,1H),6.83(s,1H),6.75(s,1H),3.76(s,3H),3.73( s,3H), 3.58(s,2H), 2.15(s,3H).
Embodiment 2
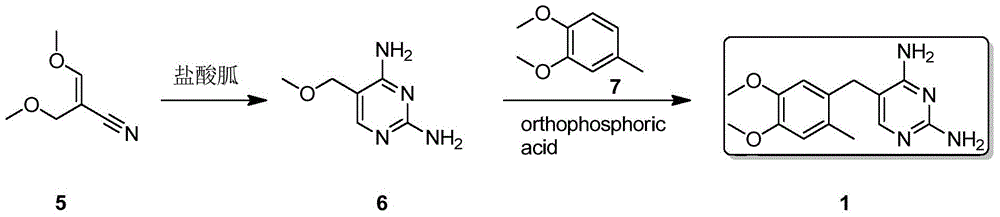
[0020] Embodiment 2, the synthesis of Omeprene
[0021] Sodium methoxide (216g, 4mol) was slowly added to methanol (900mL) solution, and then 3-methoxypropionitrile (127.5g, 1.5mol) and 4,5-dimethoxy-2-methane were added to the reaction solution Base benzaldehyde (180g, 1mol), and reacted at 55~60°C for 18 hours, then added guanidine hydrochloride (143g, 1.5mol) in batches, and reacted at 55~60°C for 18 hours, cooled to room temperature, filtered, filter cake After washing with a small amount of water, the product was dried to obtain Omepleline (200g), with a yield of 73%. White solid, melting point: 232.5~233.0℃, 1 HNMR (500MHz, d 6 -DMSO):8.2(br s,2H),7.8(br s,2H),6.86(s,1H),6.83(s,1H),6.75(s,1H),3.76(s,3H),3.73( s,3H), 3.58(s,2H), 2.15(s,3H).
Embodiment 3
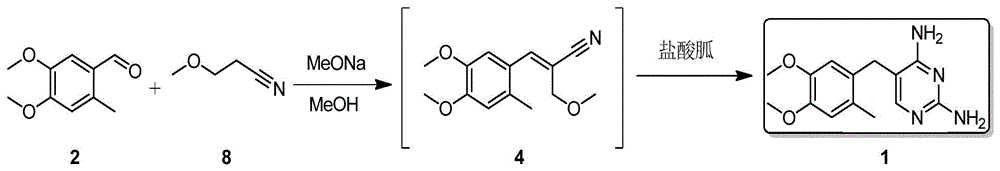
[0022] Embodiment 3, the synthesis of Omeprene
[0023] Sodium methoxide (162g, 3mol) was slowly added to methanol (900mL) solution, then 3-methoxypropionitrile (85g, 1mol) and 4,5-dimethoxy-2-methylbenzene were added to the reaction solution Formaldehyde (180g, 1mol), and react at 60-64°C for 18 hours, then add guanidine hydrochloride (95g, 1mol) in batches, and react at 60-64°C for 18 hours, cool to room temperature, filter, and wash the filter cake with a small amount of water , Obtain Omepleline (178g) after product drying, yield 65%. White solid, melting point: 32.5~233.0℃, 1 HNMR (500MHz, d 6 -DMSO):8.2(br s,2H),7.8(br s,2H),6.86(s,1H),6.83(s,1H),6.75(s,1H),3.76(s,3H),3.73( s,3H), 3.58(s,2H), 2.15(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com