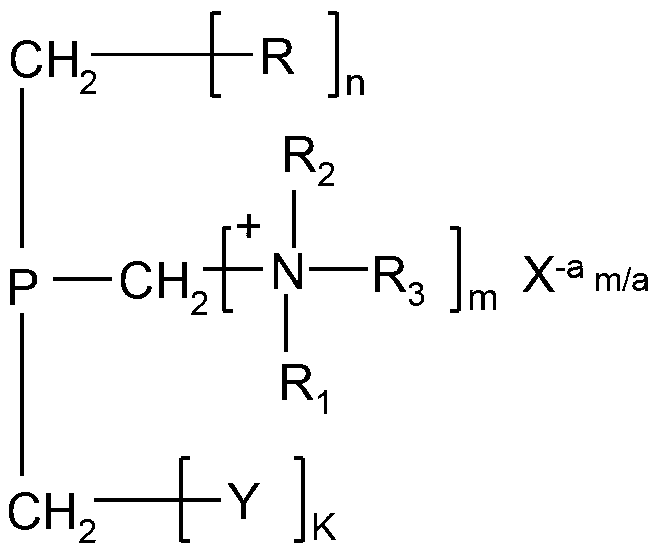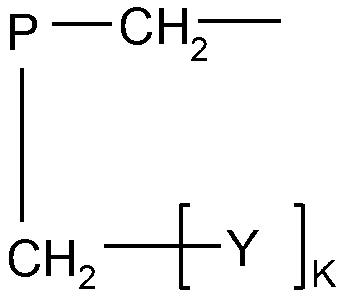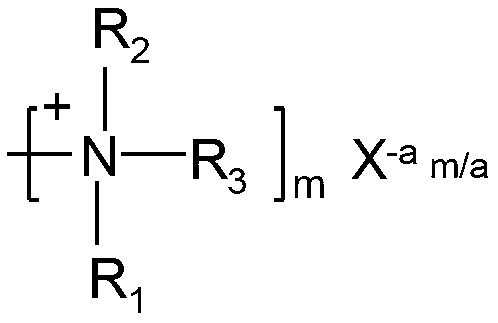Sterilization polymer containing quaternary ammonium salt and halamine or halamine precursor functional group as well as preparation method and application of sterilization polymer
A halamine functional and polymer technology, which is applied to the field of bactericidal polymers containing quaternary ammonium salts and halamine or halamine precursor functional groups and the preparation thereof, can solve the problem of low halogen utilization, poor surface hydrophilicity and limited swelling And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example is the preparation of a macroporous cross-linked resin (PSHTA3) containing quaternary ammonium salt and haloamine precursor functional groups, wherein the molar ratio of reactants 5,5-dimethylhydantoin and trimethylamine is 3: 1.
[0043] Weigh 1.77 g of 5,5-dimethylhydantoin and 1.47 g of sodium carbonate into a 250 ml round bottom flask, dissolve with 20 ml of isopropanol, add macroporous cross-linked poly-p-chloromethylstyrene resin 2.82 g, 0.91 g of 30% trimethylamine aqueous solution was added dropwise under stirring, and stirred and reacted at 60° C. for 12 hours. After the reaction, the macroporous cross-linked resin PSHTA3 containing quaternary ammonium salt and halamine precursor functional groups was obtained by filtration, washed with water for 6 times, and then dried at 45°C to constant weight to obtain 3.67 g of the product. Elemental analysis test values: C, 66.67; H, 7.876; N, 7.16. IR: 3741, 3428, 3022, 2929, 1768, 1708, 1614, 1512, 1488, ...
Embodiment 2
[0045] This example is the preparation of a macroporous cross-linked resin (PSHTA3-Cl) containing quaternary ammonium salt and haloamine functional groups.
[0046] Take by weighing 1.45 grams of PSHTA3 macroporous crosslinked resin prepared in Example 1 and place in a 50-ml beaker, add 14 milliliters of deionized water, stir in an ice bath, slowly drip 6.81 grams of 10% sodium hypochlorite solution, and simultaneously Use 1N hydrochloric acid to adjust the pH value to about 7, and continue to stir and react in the ice bath for 2 hours after the dropwise addition. After the reaction, the macroporous cross-linked resin PSHTA3-Cl containing quaternary ammonium salt and haloamine functional groups was obtained by filtration, washed with water for 6 times, and then dried at 45° C. to constant weight. Determination of oxidized chlorine (Cl) on the resin by iodometric method + ) content is 5.76%. Elemental analysis test values: C, 62.62; H, 6.980; N, 6.32. IR: 3416, 3024, 2929, 1...
Embodiment 3
[0048] This example is the preparation of a macroporous cross-linked resin (PSHTA3-Br) containing quaternary ammonium salt and haloamine functional groups.
[0049] Take by weighing 1.46 grams of PSHTA3 macroporous cross-linked resin prepared in Example 1 and place in a 50-ml beaker, add 14 ml of deionized water and 1.12 g of potassium bromide, stir in an ice bath, slowly add 10% of 6.89 grams of sodium hypochlorite solution, while using 1N hydrochloric acid to adjust the pH value to about 7, after the dropwise addition, stir and react in an ice bath for 2 hours. After the reaction, the macroporous cross-linked resin PSHTA3-Br containing quaternary ammonium salt and haloamine functional groups was obtained by filtration, washed with water for 6 times, and then dried at 45° C. to constant weight. Determination of oxidized bromine (Br) on the resin by iodometric method + ) content is 12.77%. Elemental analysis test values: C, 50.54; H, 5.619; N, 5.01. IR: 3850, 3393, 3021, 29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com