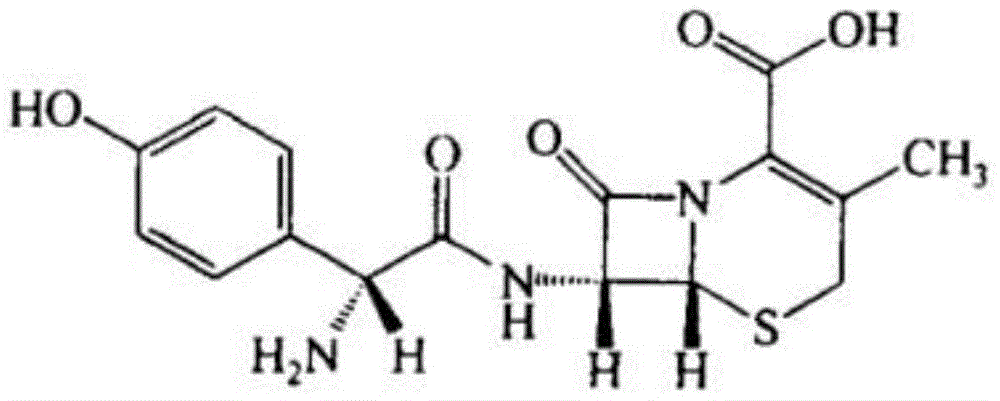
A kind of cefadroxil compound and its pharmaceutical composition
A technology of cefadroxil compound and cefadroxil hydrate, which is applied in the field of cefadroxil compound and its pharmaceutical composition, can solve the problems of poor solubility of cefadroxil, limited application, easy moisture absorption, etc., and avoid agglomeration , Uniform particle size and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
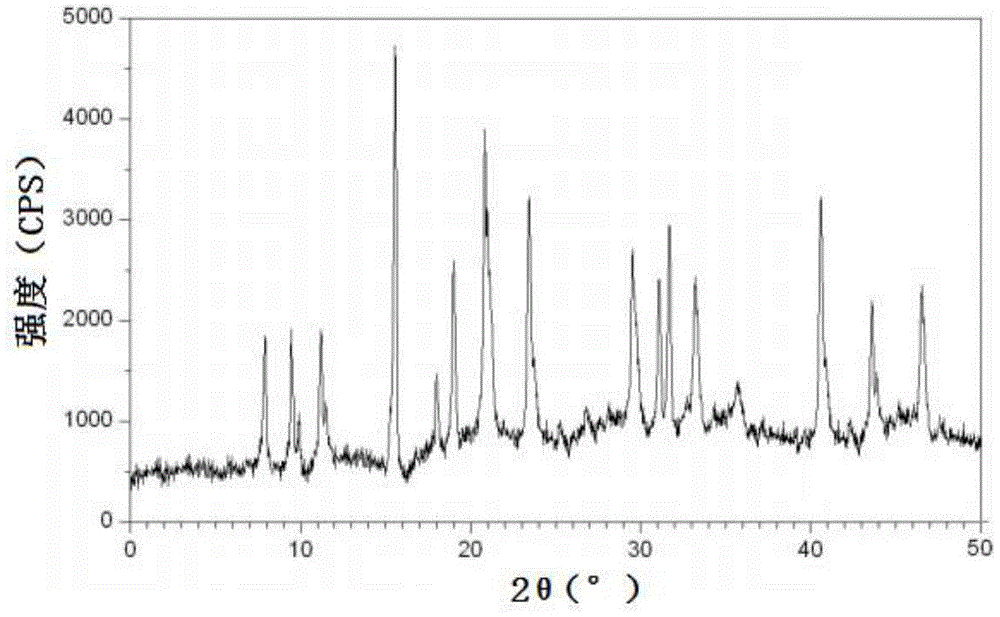
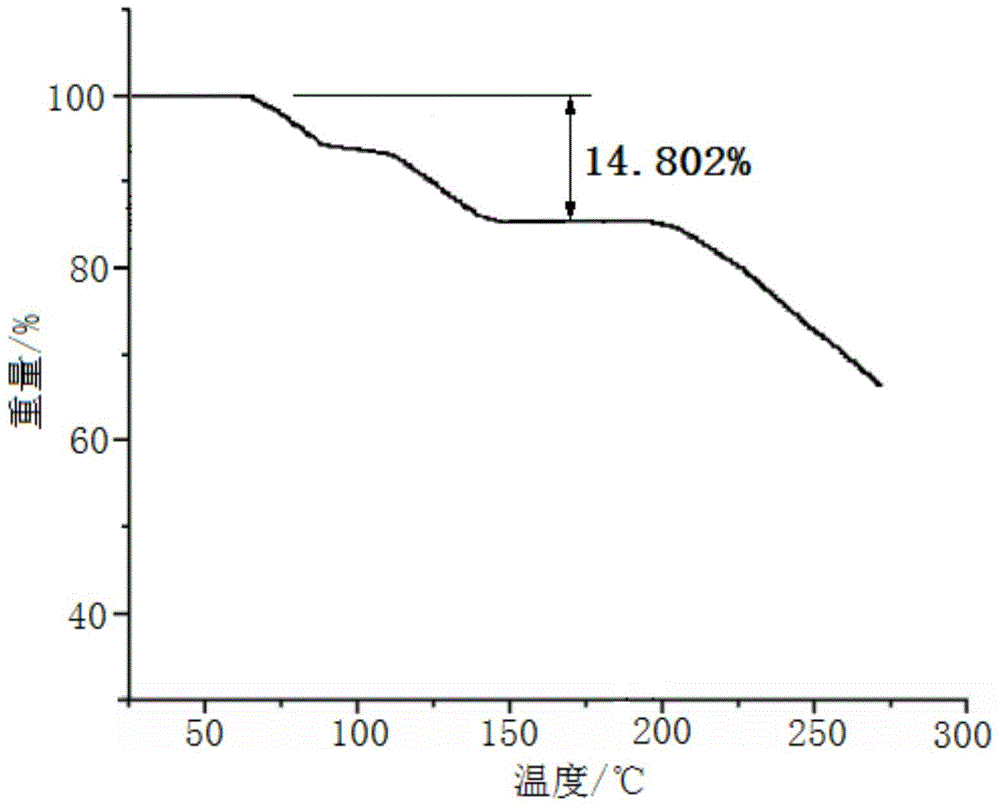
[0045] Embodiment 1, the preparation of cefadroxil hydrate crystal
[0046] 1) Dissolving the cefadroxil bulk drug in the mixed solution A made of water, acetonitrile and tetrahydrofuran, adjusting the pH value to 2.0 with hydrochloric acid to obtain the water / acetonitrile / tetrahydrofuran solution of cefadroxil, wherein the cefadroxil bulk drug The weight to volume ratio with mixed solution A (the volume ratio of water, acetonitrile and tetrahydrofuran in mixed solution A is 10:3:1) is 1g:50ml;
[0047] 2) Under a sound field with a frequency of 15KHz and an output power of 30W, add dropwise at 50ml / min a mixed solution B made of acetone and ether at 2°C under stirring at a stirring speed of 100 rpm (in the mixed solution B The volume ratio of acetone and ether is 1:3), the volume of the mixed solution B of acetone and ether added dropwise is 3 times of the water / acetonitrile / tetrahydrofuran solution of cefadroxil; after the dropwise addition, turn off the sound field and let ...
Embodiment 2
[0050] Embodiment 2, the preparation of cefadroxil hydrate crystal
[0051] 1) Dissolving the cefadroxil bulk drug in the mixed solution A made of water, acetonitrile and tetrahydrofuran, adjusting the pH value to 2.4 with hydrochloric acid to obtain the water / acetonitrile / tetrahydrofuran solution of cefadroxil, wherein the cefadroxil bulk drug The weight to volume ratio of mixed solution A (the volume ratio of water, acetonitrile and tetrahydrofuran in mixed solution A is 10:6:2) is 1g:100ml;
[0052] 2) Under a sound field with a frequency of 22KHz and an output power of 60W, add dropwise at 100ml / min the mixed solution B (in the mixed solution B) made of acetone and ether at 8°C under stirring at a stirring speed of 120 rpm The volume ratio of acetone and ether is 1:6), the volume of the mixed solution B of acetone and ether added dropwise is 8 times that of the water / acetonitrile / tetrahydrofuran solution of cefadroxil; after the dropwise addition, turn off the sound field ...
Embodiment 3
[0055] Embodiment 3, the preparation of cefadroxil hydrate crystal
[0056] 1) Dissolving the cefadroxil bulk drug in the mixed solution A made of water, acetonitrile and tetrahydrofuran, adjusting the pH value to 2.2 with hydrochloric acid to obtain the water / acetonitrile / tetrahydrofuran solution of cefadroxil, wherein the cefadroxil bulk drug The weight to volume ratio with mixed solution A (the volume ratio of water, acetonitrile and tetrahydrofuran in mixed solution A is 10:5:3) is 1g:80ml;
[0057] 2) Under a sound field with a frequency of 20KHz and an output power of 50W, add dropwise at 80ml / min a mixed solution B (in the mixed solution B) made of acetone and ether at 5°C under stirring at a stirring speed of 110 rpm. The volume ratio of acetone and ether is 1:5), the volume of the mixed solution B of acetone and ether added dropwise is 5 times that of the water / acetonitrile / tetrahydrofuran solution of cefadroxil; after the dropwise addition, turn off the sound field a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com