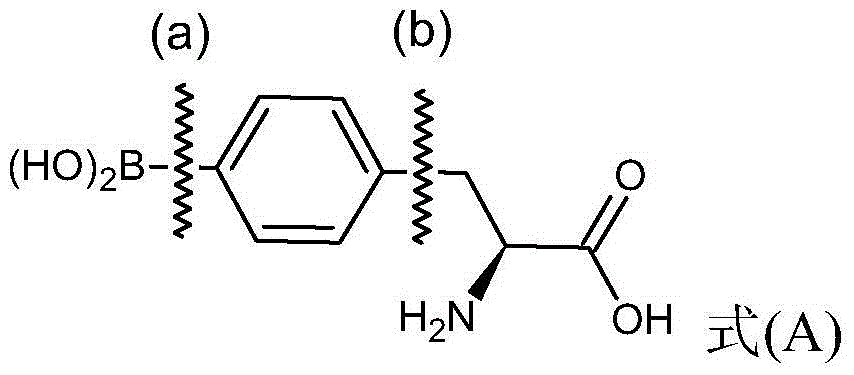
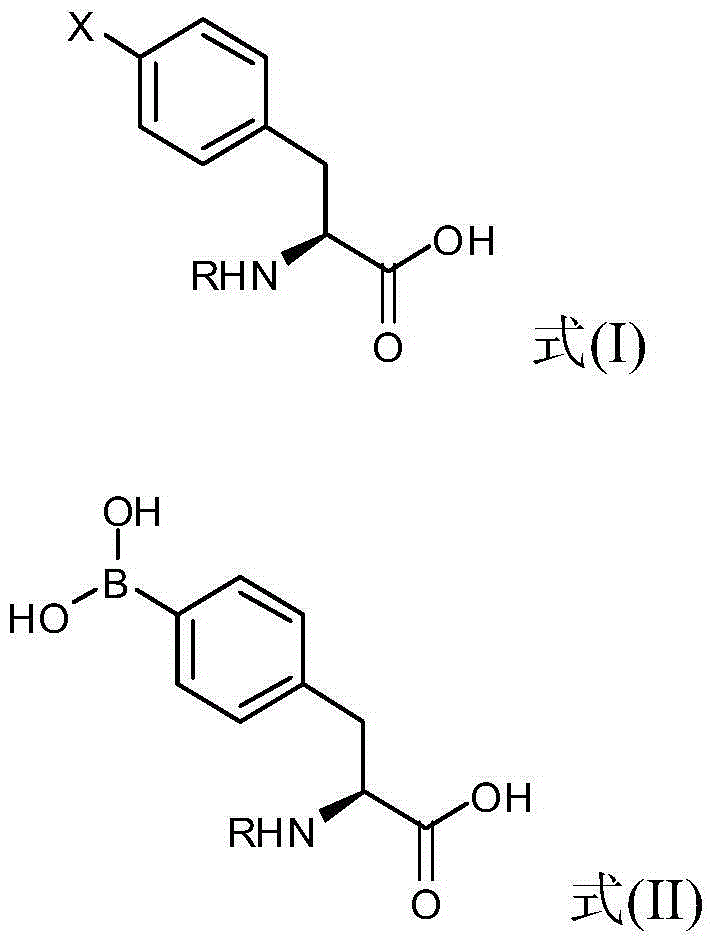
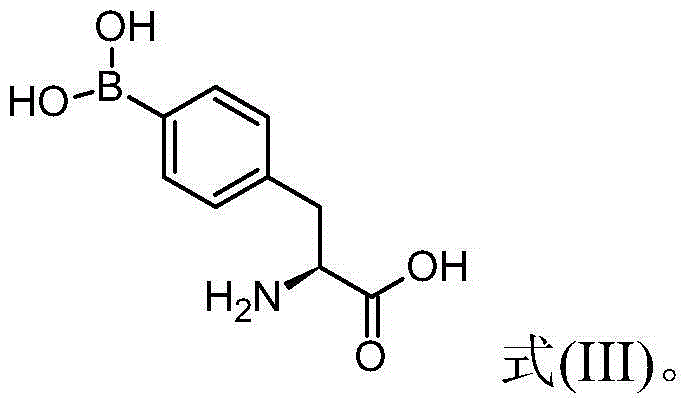
4 - dihydroxy boryl - l-phenylalanine preparation method
A technology of hydroxyboronyl phenylalanine and halophenylalanine, which is applied in the field of preparation of 4-dihydroxyboronyl-L-phenylalanine, and can solve the problem of low L-BPA yield, inapplicability, etc. problem, to achieve the effect of high cost efficiency, saving time and shortening the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] (1) Preparation of (S)-N-tert-butoxycarbonyl-4-dihydroxyboryl-phenylalanine from (S)-N-tert-butoxycarbonyl-4-iodophenylalanine
[0101] Please refer to the following reaction formula (I), which is the reaction of (S)-N-tert-butoxycarbonyl-4-iodophenylalanine with tributyl borate to synthesize (S)-N-tert-butoxycarbonyl- The chemical reaction formula of 4-dihydroxyborylphenylalanine.
[0102]
[0103] First, a 1-liter three-necked bottle equipped with a mechanical stirrer, a thermometer, and a nitrogen gas inlet joint sealed with a rubber gasket was prepared. Inject 150 milliliters of 2-methyltetrahydrofuran into the three-necked flask, and then add 24.7 millimoles (10.0 grams, purity 96.8%) of (S)-N-tert-butoxycarbonyl-4-iodophenylalanine, The two were uniformly stirred to form a solution; then, 77.8 mmol (17.9 g, 21 ml) of tributyl borate was added to the solution to form a mixed solution.
[0104] Next, the mixed solution was cooled to between -85°C and -76°C, and...
Embodiment 2
[0132] (1) Prepare (S)-N-tert-butoxycarbonyl-4-( 10 B) Dihydroxyboryl phenylalanine
[0133]First, a 3-liter three-necked bottle equipped with a mechanical stirrer, a thermometer, and a nitrogen inlet joint sealed with a rubber gasket was prepared. Inject 750 milliliters of 2-methyltetrahydrofuran into the three-necked flask, then add 128 millimoles (50.0 grams, 100% purity) of (S)-N-tert-butoxycarbonyl-4-iodophenylalanine, And the two were uniformly stirred to form a solution; after that, 393 mmol (90.1 g, 106 ml) of 10 Tributyl borate was added to the solution to form a mixed solution.
[0134] Next, the mixed solution was cooled to between -85°C and -76°C, and a 1.6M (600 mmol, 375 ml) hexane solution of n-butyllithium was added dropwise to the mixed solution over 3 hours to A reaction mixture is obtained.
[0135] Then, the reaction mixture was stirred at -80°C for 0.5 hours, and a small amount of the reaction mixture sample was quickly added to water and analyzed by H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com