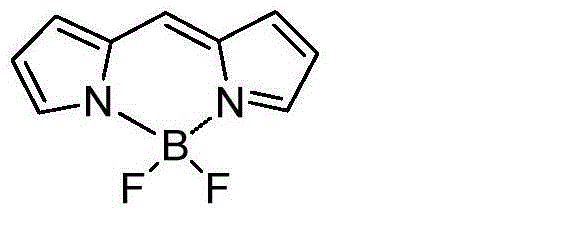
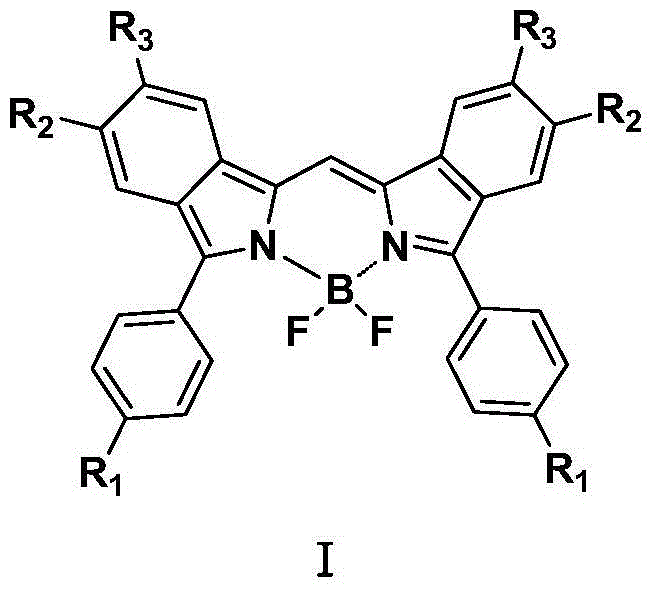
Fluoro-boron diisoindole compounds and preparation method thereof
A compound, the technology of isoindole, which is applied in the field of fluoroborate diisoindole compounds and their preparation, can solve the problems of affecting biological activity, lengthy synthetic route, and increased difficulty of compound synthesis, and achieve the increase of π-bond conjugation range , the effect of changing photochemical and physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
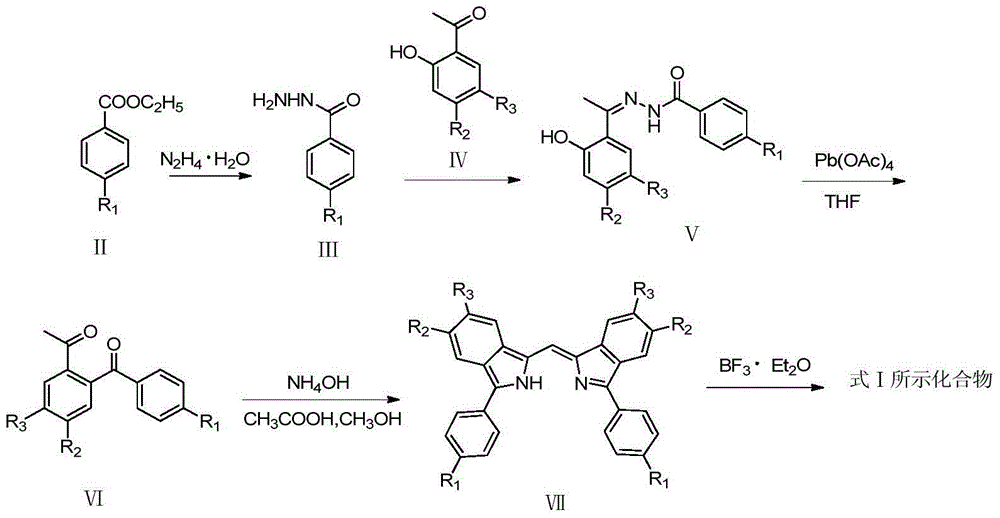
[0025] The method for preparing the compound shown in formula I provided by the present invention specifically comprises the following steps:
[0026] (1) Synthesis of 4-substituted phenylhydrazine (compound shown in formula III)
[0027] Add 20~25mmol of ethyl 4-substituted benzoate (compound shown in formula II), 50~60mmol of hydrazine hydrate (N 2 h 4 ·H 2 O), add 50~60mL dehydrated alcohol simultaneously as solvent, stir, after 75~80 ℃ heat reflux 6~7h, remove solvent, have precipitation to separate out, filter, dry, obtain 4-substituted phenylhydrazine (formula III indicated compounds);
[0028] (2) Synthesis of N-[1-(aryl)ethylidene]aromatic hydrazide (compound shown in formula V)
[0029] Add 15~20mmol of 4-substituted benzohydrazide prepared in step (1), 18~20mmol of 4,5-substituted 2-hydroxyacetophenone (compound shown in formula IV) into the reaction bottle, and add 50~60mL of dehydrated ethanol, stirred, heated to reflux at 75-80°C for 48-50h, the solvent was r...
Embodiment 1
[0039] 1,1'-bis(4,4'-fluoro)phenyl-3,3'-fluoroborodiisoindolylidene (IA)
[0040]
[0041] (1) Synthesis of 4-fluorobenzoylhydrazide
[0042] Add 3.36g (20mmol) ethyl p-fluorobenzoate, 5.00g (50mmol) hydrazine hydrate (N2H4 H2O) and 50mL absolute ethanol to a 100mL reaction flask, stir, heat and reflux at 75-80°C for 6h, then distill off the ethanol, White needle-like crystals were precipitated, filtered, washed, and dried to obtain 2.78 g of 4-fluorobenzoic hydrazide, yield: 90.3%, melting point: 163°C.
[0043] (2) Synthesis of N-[1-(2-hydroxyphenyl)ethylidene]-4-fluorobenzohydrazide
[0044]Add 2.31g (15mmol) of 4-fluorobenzoic hydrazide, 2.44g (18mmol) of 2-hydroxyacetophenone, and 50mL of absolute ethanol into a 100mL reaction bottle, stir, heat and reflux at 75-80°C for 48 hours, and wait for the reaction After the end, a yellow solid precipitated, filtered, washed, and dried to obtain 3.35 g of N-[1-(2-hydroxyphenyl)ethylidene]-4-fluorobenzohydrazide, yield: 82.2%,...
Embodiment 2
[0054] 1,1'-bis(4,4'-chloro)phenyl-3,3'-fluoroborodiisoindolylidene (IB)
[0055]
[0056] Except that the ethyl p-fluorobenzoate in step (1) of Example 1 was replaced with ethyl p-chlorobenzoate, other conditions were the same as in Example 1 to obtain the title compound.
[0057] 1 H NMR (400MHz, CDCl 3 ),δ:7.84(d,2H,J=8.4Hz),7.77(s,1H),7.79(d,4H,J=8.4Hz),7.52 (d,2H,J=8.0Hz),7.48~7.39 (m,6H),7.20~7.20(m,2H).
[0058] HRESI-MS535.0726.([M+Na] + , calcd for C 29 h 17 BF 2 N 2 Cl 2 Na:535.0722).UV:λmax=648nm.Fluorescence:λem=673nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com