Application of f52-2 protein and its coding gene and hydrolyzed xylan
A protein and coding region technology, applied in the fields of application, hydrolase, genetic engineering, etc., can solve problems that limit the efficient development of the biofuel industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Cloning of embodiment 1, F52-2 gene
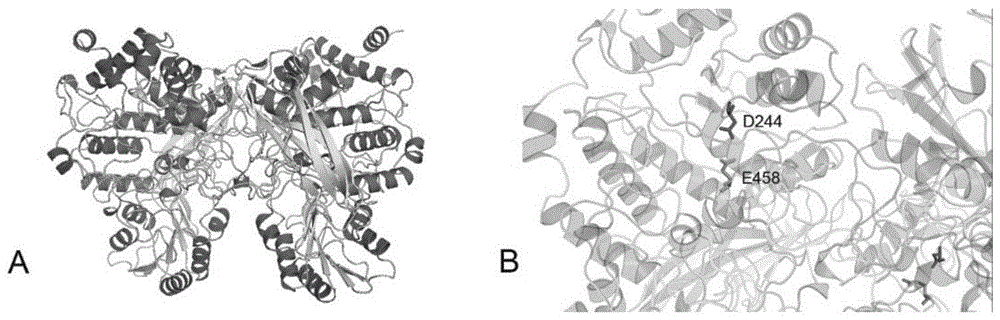
[0041] In the previous study, the metagenomic library of the dry fermented sludge system was constructed using the Fosmid vector. The ORF was predicted by sequencing and softberry, and the NCBI and Pfam databases were annotated. Get the F52-2 gene, the nucleotide sequence of this gene is the 11th-2170th nucleotide from the 5' end of sequence 1 in the sequence listing, the protein encoded by it is named F52-2, and its amino acid sequence is the sequence in the sequence listing 2 Amino acids 1-719 from the N' terminal. After comparison, the F52-2 protein has the highest similarity of 63% with the β-xylosidase from Clostridium stercorarium, and it is predicted to be a β-xylosidase.
Embodiment 2
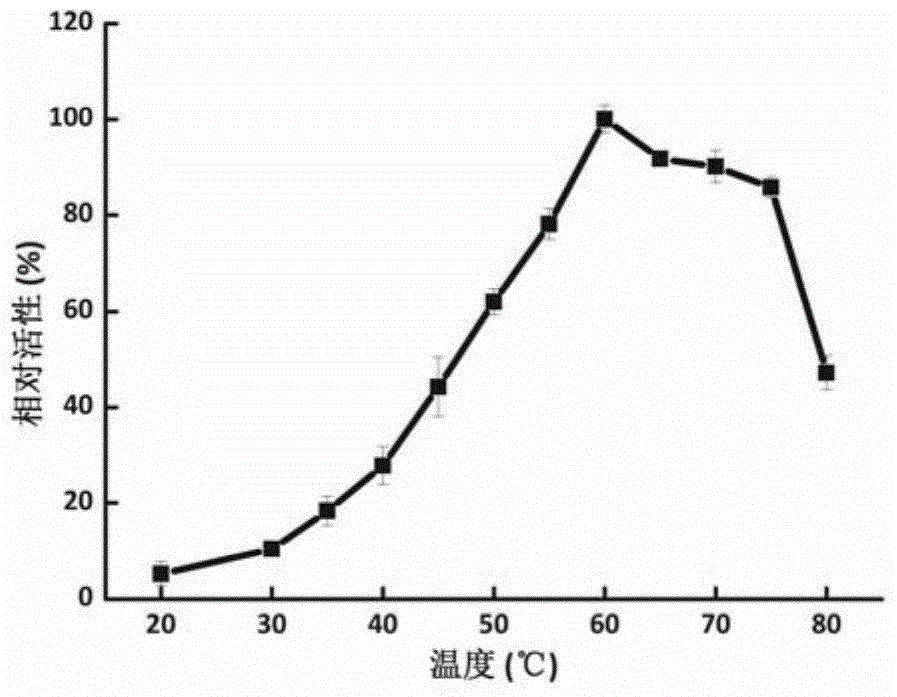
[0042] Embodiment 2, the application of F52-2 as β-xylosidase
[0043] 1. Construction of recombinant vector
[0044] Using the artificially synthesized sequence 1 as a template, using F52-2F and F52-2R as primers, and using TAKARA's PrimeStar high-fidelity enzyme to perform PCR amplification, a 2179bp PCR product was obtained, and its nucleotide sequence was sequence 1.
[0045] F52-2F:5'-GGAATTC CATATG CTTTTCCAGCGAAGTGACCTGC-3' contains Nde I restriction site
[0046] F52-2R:5'-CCC AAGCTT TCAGCGGGGCAGCTCCAG-3' contains a Hind III restriction site
[0047] The above PCR amplification system is as follows:
[0048]
[0049] The PCR program is as follows:
[0050] Pre-denaturation at 94°C for 1 min
[0051] 98°C 10s
[0052] 68°C 120s
[0053] Back to 2, 24 cycles
[0054] 72℃ 5min
[0055] end
[0056] The above PCR product was double digested with Nde I and Hind III, and the resulting digested product was ligated with the pET-28a vector (Novagen pET-28a DNA Ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com