Method of generating 1,2,4-butantriol by in vitro enzyme reaction and application thereof
A technology of butanetriol and in vitro enzymes is applied in the field of bioengineering to achieve the effects of improving activity, shortening production cycle and increasing product quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
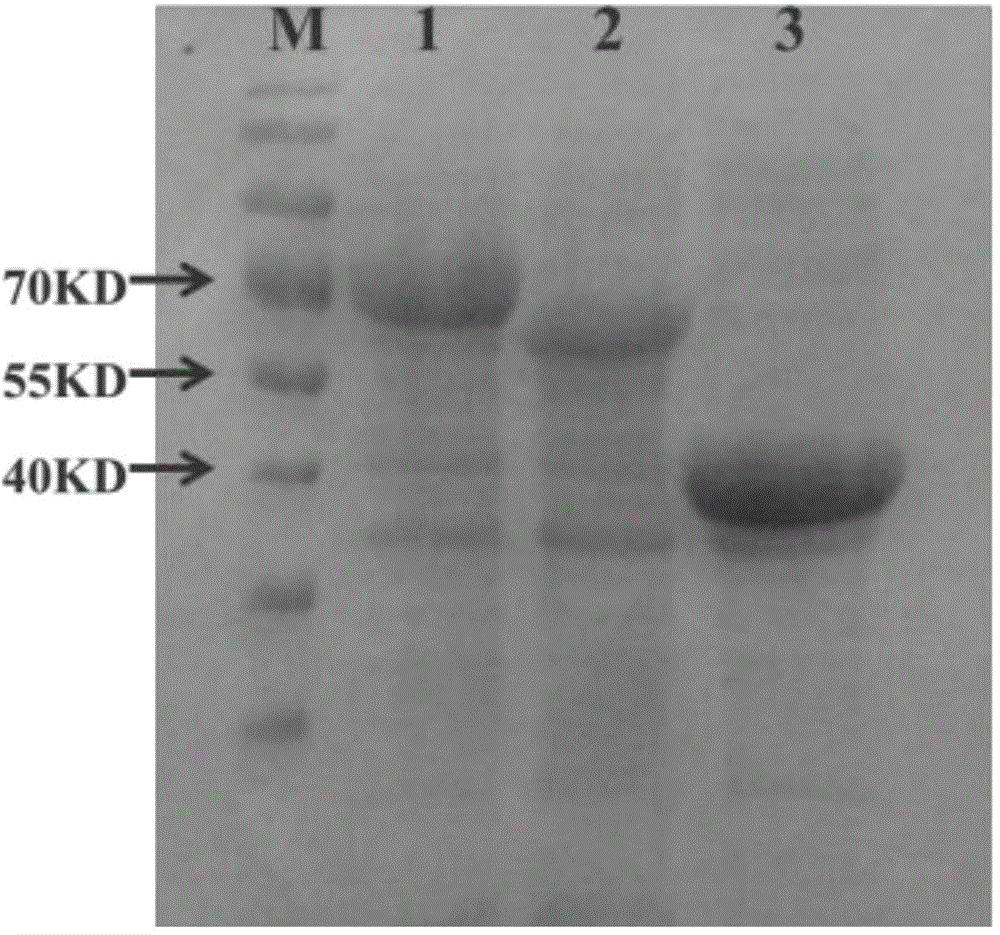
[0037] Preparation of crude enzyme solution of enzymes related to 1,2,4-butanetriol anabolic metabolism, the specific steps are as follows:
[0038] (1) Use primers at the 5' end and 3' end of the yjhG gene to introduce restriction sites (NcoI and EcoRI), carry out double enzyme digestion on the yjhG gene and pET-30a(+), and then connect the yjhG gene to pET-30a ( +) on the carrier;
[0039] (2) Use primers at the 5' end and 3' end of the mdlC gene to introduce restriction sites (NcoI and EcoRI), carry out double enzyme digestion on the mdlC gene and pET-30a(+), and then connect the mdlC gene to pET-30a ( +) on the carrier;
[0040] (3) Utilize adhP gene 5' and 3' end primers to introduce restriction sites (NcoI and EcoRI), carry out double enzyme digestion to adhP gene and pET-30a (+), then connect adhP gene to pET-30a ( +) on the carrier;
[0041] (4) Transform the corresponding three recombinant plasmids constructed in steps (1), (2), and (3) into Escherichia coli BL21 (...
Embodiment 2
[0044] The in vitro reaction of enzymes related to 1,2,4-butanetriol synthesis and metabolism, the specific steps are as follows:
[0045] (1) Take 100ml of E. coli culture solution expressing the above three enzymes at 4200rpm, centrifuge at 4°C for 5min, discard the supernatant, wash twice with 50mM pH 7.0 Hepesbuffer, and finally resuspend with 5ml Hepesbuffer; then break it with a sonicator (22kHz, 150w), crush for 30min, centrifuge the crushed solution at 15000rpm at 4°C for 10min, separate the supernatant from the precipitate, keep the supernatant, add 10% glycerol, and measure the protein content according to the kit method.
[0046] (2) The D-xylonate dehydratase, 2-ketoacid decarboxylase, and alcohol dehydrogenase prepared in step (1) are added to the reaction system, and the concentration of D-xylonate dehydratase is kept at 0.39 mg / ml, 2- The concentration of ketoacid decarboxylase is 0.05mg / ml, the concentration of alcohol dehydrogenase is 0.39mg / ml, and other reac...
Embodiment 3
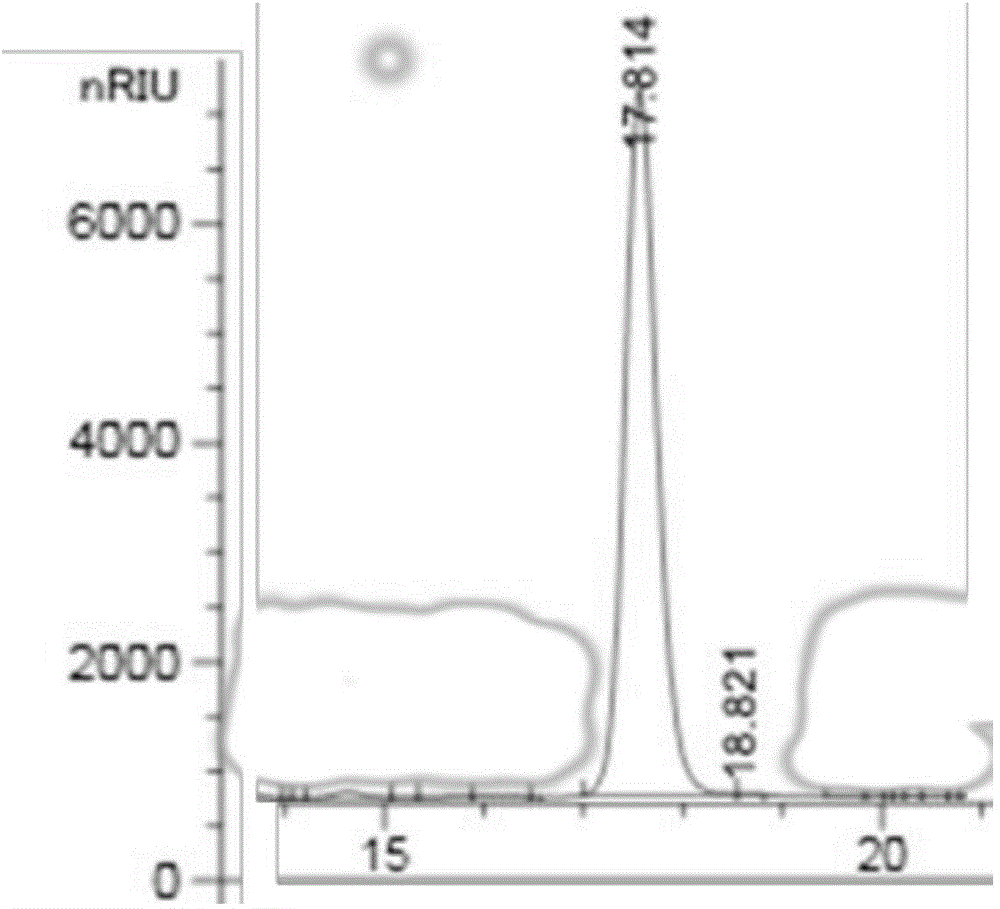
[0049] Intermediate level 1,2,4-butanetriol is synthesized in vitro, and the specific steps are as follows:
[0050] (1) Take 100ml of E. coli culture solution expressing the above three enzymes at 4200rpm, centrifuge at 4°C for 5min, discard the supernatant, wash twice with 50mM pH 7.0 Hepes buffer, and finally resuspend with 5ml Hepes buffer; Instrument crushing (22kHz, 150w), crushing 30min, crushing liquid 15000rpm 4 ℃ centrifugation 10min, supernatant and precipitate were separated, supernatant was retained, 10% glycerol was added, and the protein content was determined according to the kit method.
[0051] (2) The D-xylonate dehydratase, 2-ketoacid decarboxylase, and alcohol dehydrogenase prepared in step (1) are added to the reaction system, and the concentration of D-xylonate dehydratase is kept at 0.39 mg / ml, 2- The concentration of ketoacid decarboxylase is 0.5mg / ml, the concentration of alcohol dehydrogenase is 0.39mg / ml, and other reaction substances are added, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com