Composite catalyst of air electrode of lithium-air cell
A composite catalyst, lithium-air battery technology, applied in battery electrodes, fuel cell-type half-cells, primary battery-type half-cells, circuits, etc., can solve problems such as poor stability, poor stability performance, and large voltage polarization Achieve low charge-discharge polarization and cycle stability, improve dispersion and stability, and improve overall performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
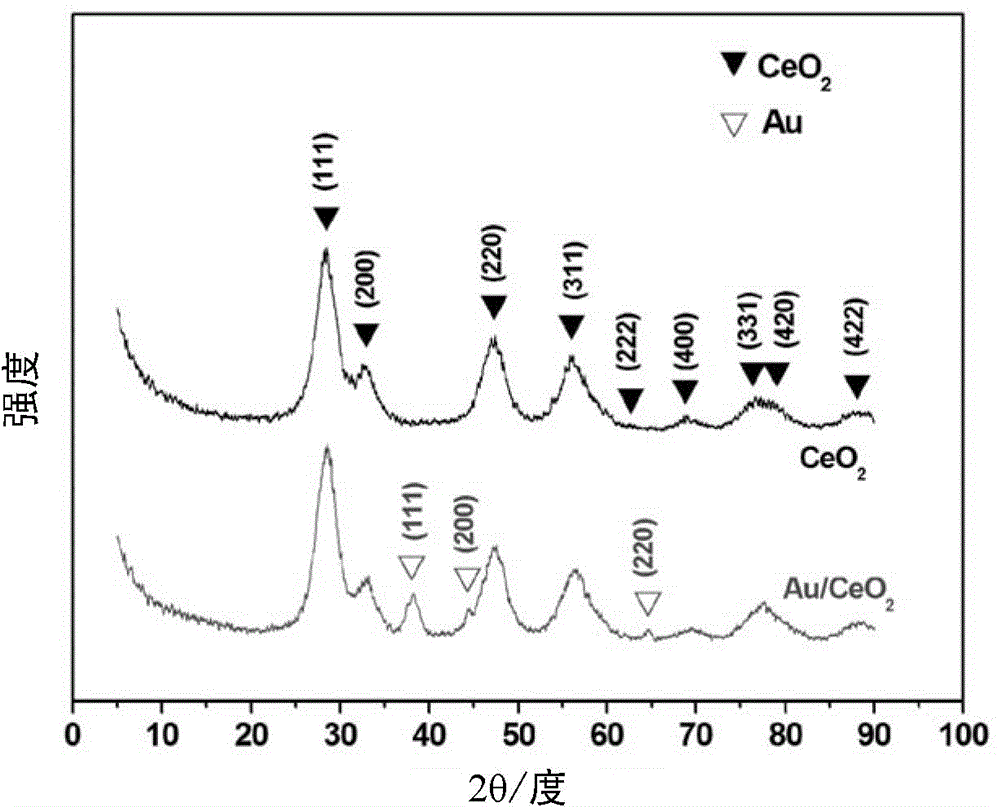
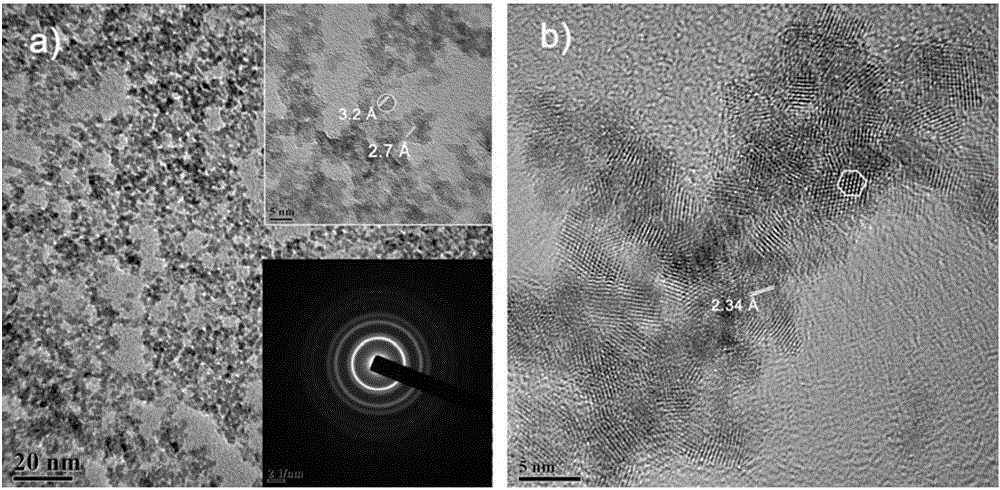
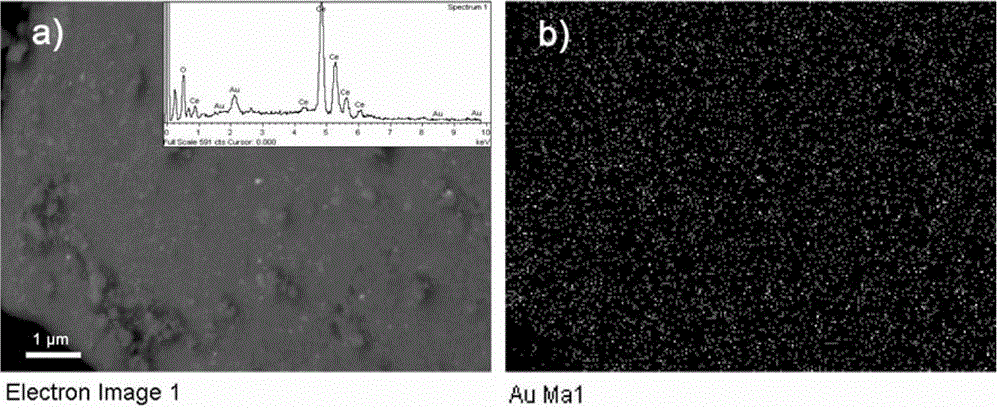
[0098] with HAuCl 4 As a precursor, the CeO obtained in Comparative Example 3 2 Nanocrystalline (diameter 4nm, specific surface area 180m 2 / g) dispersed in water to form a sol, adjust the pH to 10, so that Au can be loaded on CeO in situ 2 On nanocrystals, a composite catalyst (Au / CeO 2 ). Among them, Au is the metal catalyst, and CeO 2 Nanocrystals are the support. figure 1 Be the support body CeO of comparative example 3 2 And the metal catalyst Au of the present embodiment is on the support body CeO 2 Composite catalyst Au / CeO obtained after in situ loading 2 The X-ray diffraction pattern, see figure 1 It can be seen that Au has been loaded to CeO 2 on nanocrystals. figure 2 The support body CeO obtained for Comparative Example 3 2 And the composite catalyst Au / CeO in the present embodiment 2 The transmission electron microscope pictures, see figure 2 It can be seen that the morphology of Au is about 3nm particles, and the loading amount is 3wt%. image 3 F...
Embodiment 2
[0100] with HAuCl 4 For the precursor, the Y obtained in Comparative Example 4 2 o 3 Nanocrystalline (diameter 6nm, specific surface area 70m 2 / g) is dispersed in the sol formed by water, and the pH is adjusted to 10, so that Au can be loaded on Y in situ 2 o 3 On the nanocrystal, the composite catalyst (Au / Y 2 o 3 ). where Au is the metal catalyst, and Y 2 o 3 Nanocrystals are the support. The morphology of Au is about 5nm particles, and the loading amount is 2.5wt%. in Au / Y 2 o 3 As a catalyst, acetylene black and polyvinylidene fluoride (PVDF) are in a mass ratio of 19:11:15, in the method of Comparative Example 1, and the battery assembly and test conditions are the same as those of Comparative Example 1. The measurement results are shown in Table 1. As can be seen from the data in Table 1, compared with Comparative Example 1, Comparative Example 2 and Comparative Example 4, containing the composite catalyst Au / Y 2 o 3 The air electrode (Au accounts for 1.0...
Embodiment 3
[0102] with HAuCl 4 As a precursor, the TiO obtained in Comparative Example 5 2 (Degussa P25, diameter 25nm, specific surface area 55m 2 / g) in the dispersion solution, adjust the pH to 9, so that Au can be loaded on TiO in situ 2 On (Au / TiO 2 ). Among them, Au is the metal catalyst, and TiO 2 Nanocrystals are the support. The morphology of Au is about 4nm particles, and the loading amount is 1.5wt%. With Au / TiO 2 As a catalyst, acetylene black and polyvinylidene fluoride (PVDF) are in a mass ratio of 19:11:15, in the method of Comparative Example 1, and the battery assembly and test conditions are the same as those of Comparative Example 1. The measurement results are shown in Table 1. As can be seen from the data in Table 1, compared with Comparative Example 1, Comparative Example 2 and Comparative Example 5, containing the composite catalyst Au / TiO 2 The air electrode (Au accounts for 0.63wt% of the total) makes the lithium-air battery have a non-catalytic electrod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com