Preparation method of bi-combined inactivated vaccine
A dual inactivated vaccine and inactivation technology, applied in the field of vaccines, can solve the problems of scattered virus and difficult virus cultivation, and achieve the effects of convenient immunity, reduced stress response and high titer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
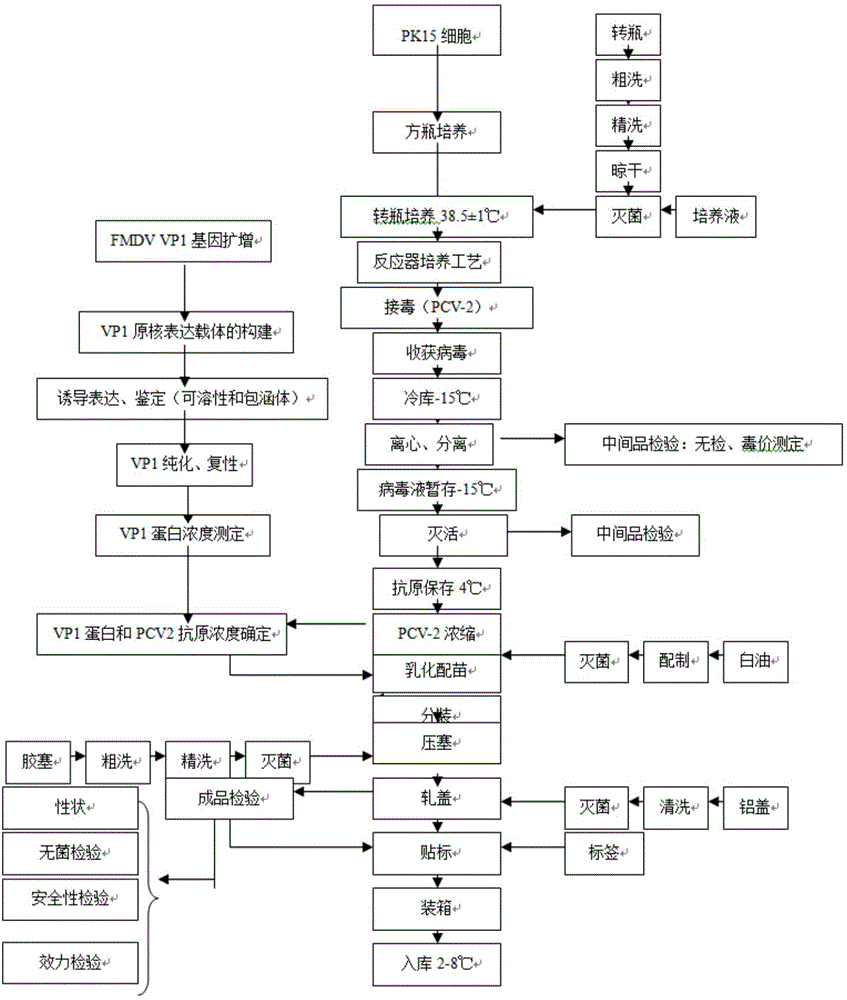
Image
Examples
Embodiment 1
[0033] Example 1 Porcine circovirus type 2, porcine foot-and-mouth disease VP1 subunit dual inactivated vaccine
[0034] 1 Preparation of poisonous seeds for production
[0035] 1.1 Preparation of PCV2ZJ / C strain: Dilute the virus seeds appropriately with virus diluent (serum-free MEM medium), inoculate them on PK-15 cell culture at a multiplicity of infection (M.O.I.) of 0.01, absorb at 37°C for 30 minutes, add MEM cell maintenance solution with 3% (v / v) calf serum and 1mmol / L D-glucosamine hydrochloride, cultured at 37°C for 5 days, repeated freezing and thawing 3 times, harvested virus, virus titer ≥ 10 7.0 TCID 50 / ml.
[0036] 1.2 Activation of VP1 protein expression strains: Streak the preserved prokaryotic expression strains onto a Kan+ resistant culture plate and culture overnight at 37°C. Pick a single colony and insert it into 10mL liquid LB medium containing Kan+, shake and culture at 220r / min at 37°C for 11-16h, and use it as a strain for inducing expression.
...
Embodiment 2
[0080] Embodiment 2 FMDV VP1 subunit, PCV2 dual inactivated vaccine and the immune effect comparison of using two kinds of vaccines (swine foot-and-mouth disease O type synthetic peptide vaccine (polypeptide 2570+7309) and PCV2 inactivated vaccine) to immunize piglets alone
[0081] 1. Materials
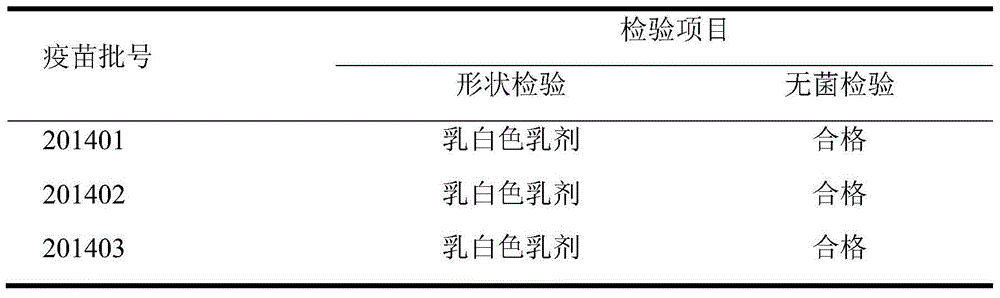
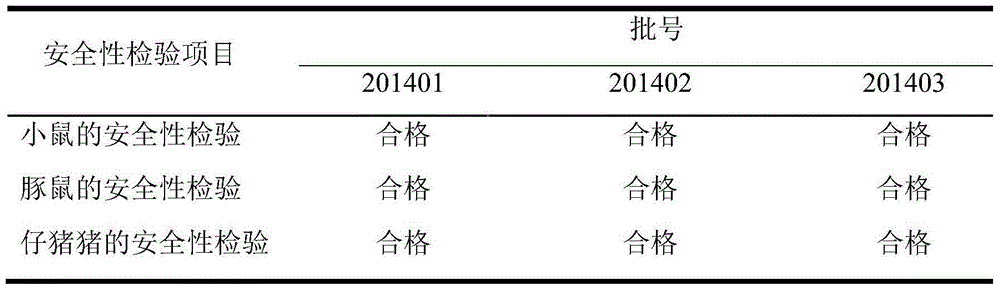
[0082] Pig O-type foot-and-mouth disease VP1 subunit, PCV2 dual inactivated vaccine, select the laboratory product (lot number is 201401) in the embodiment 1 for use; Produced by the company (batch number is XXXX), the virus content is at least 10% before inactivation 7.0 TCID 50 / ml; FMD O-type synthetic peptide vaccine (polypeptide 2570+7309), purchased from Shanghai Shenlian Biological Co., Ltd. (batch number XXXX), containing at least 25ug / ml of FMD synthetic peptides.
[0083] 2. Design of animal experiments
[0084] Select 90 weaned piglets aged 21 to 28 days and divide them into 6 groups, 15 pigs in each group; each pig in the 1st and 2nd groups was injected intramuscularly ...
Embodiment 3
[0090] 1. A method for preparing a double inactivated vaccine, characterized in that it comprises the following steps:
[0091] 1) Take the PCV2ZJ / C strain to infect the host cells for virus amplification to ensure that the virus titer is ≥ 10 7.0 TCID 50 / ml, harvest cytovenom;
[0092] 2) The cell venom obtained in step 1) is clarified and concentrated by using a hollow fiber filter column;
[0093] 3) adding β-propiolactone to inactivate the concentrated virus in step 2) in a stirring state at a ratio of 0.1 to 0.3% (v / v), to obtain the first antigen stock solution;
[0094] 4) The pET28a-FMDV VP1 strain was cultured and amplified, and then 0.1-1 mmol / L IPTG was added to the culture system for induction. The induction time was 4-6 hours, and the induction temperature was 25-37°C. wall, collecting inclusion bodies;
[0095] 5) Use CAPS lysate or urea lysate to resuspend the inclusion bodies obtained in step 4) at a concentration of 15-25 mg / mL, collect the supernatant, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com