Catalytic denitrification method
A catalytic denitration and catalyst technology, applied in the field of catalytic denitration, can solve the problems of complex process and low NO removal rate, and achieve the effects of simple process, low operating cost and low operating temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of Composite Metal Oxide Catalyst Cu(0.4)-NiO by Co-precipitation x , Ni(0.4)-FeO x , Cu(1)-NiO x and Cu(2.5)-NiO x (such as Cu(0.4)-NiO x It represents that the stoichiometric ratio of Cu and Ni in this composite metal oxide is 0.4:1). According to the stoichiometric ratio, take the required metal nitrate solution (concentration is 1mol / L) and mix evenly, slowly drop the mixed metal salt solution into 1mol / L Na 2 CO 3 (control solution temperature is 50 ℃) carry out precipitation reaction. When the pH of the control end point was 8, the dropwise addition was stopped. Stirring and aging were continued for 3 h at the same temperature. Then wash, filter, and dry at 60°C for 5h to obtain a catalyst precursor, which is calcined in a muffle furnace at 400°C for 5h to obtain the desired stoichiometric ratio of Cu(0.4)-NiO x , Ni(0.4)-FeO x , Cu(1)-NiO x and Cu(2.5)-NiO x Composite metal oxide catalyst samples.
Embodiment 2
[0022] The method of the present invention includes filling the reaction tube of the microwave catalytic reactor device with catalyst to form a microwave catalytic reaction bed, and the gas to be treated undergoes a gas-solid phase reaction when passing through the microwave catalytic reaction bed for denitrification treatment; the composite metal oxide The catalyst includes Cu-Ni composite metal oxide and Ni-Fe composite metal oxide, composed of Cu(0.4)-NiO x , Ni(0.4)-FeO x , Cu(1)-NiO x and Cu(2.5)-NiO x ; The catalyst is filled in the reaction tube of the microwave catalytic reactor device, when the treated gas passes through the microwave catalytic reaction bed, the NO in the gas is directly decomposed and reacted to generate environmentally friendly N 2 and O 2 , in order to achieve the purpose of direct denitrification.
[0023] In this embodiment, the waste gas provided by Dalian Date Gas Co., Ltd. is N 2 and NO, the NO concentration is 1000ppm. NO x Analyzer is...
Embodiment 3
[0030] It is basically the same as Example 2, except that the content of oxygen in the intake air is changed to 0-10%.
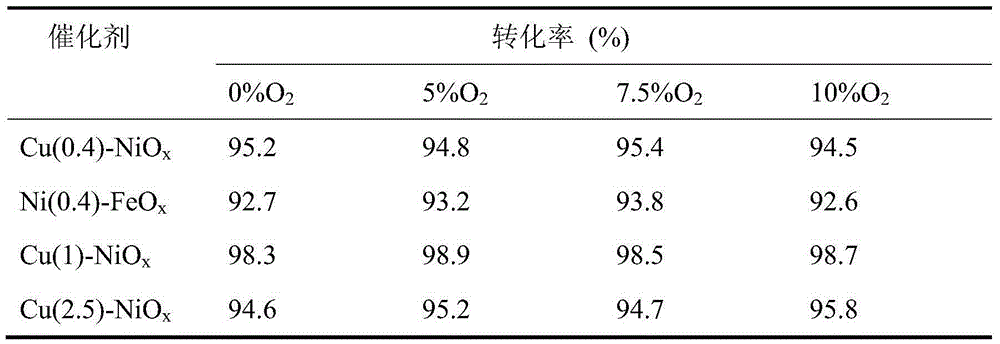
[0031] The packed catalyst is Cu(0.4)-NiO x , Ni(0.4)-FeO x , Cu(1)-NiO x and Cu(2.5)-NiO x . The loading amount of the catalyst is 2g respectively, and the mesh number is 20-60 mesh. The intake NO concentration is 1000ppm, the oxygen content in the intake air is changed to 0%, 5%, 7.5%, and 10%, the residence time of the gas in the microwave catalytic reaction bed is 1s, and the reaction pressure is normal pressure. The microwave power was adjusted to maintain the temperature of the catalyst bed at 250°C, and the oxidation resistance of the catalyst in the microwave catalytic decomposition of NO was investigated. The results are shown in Table 2.
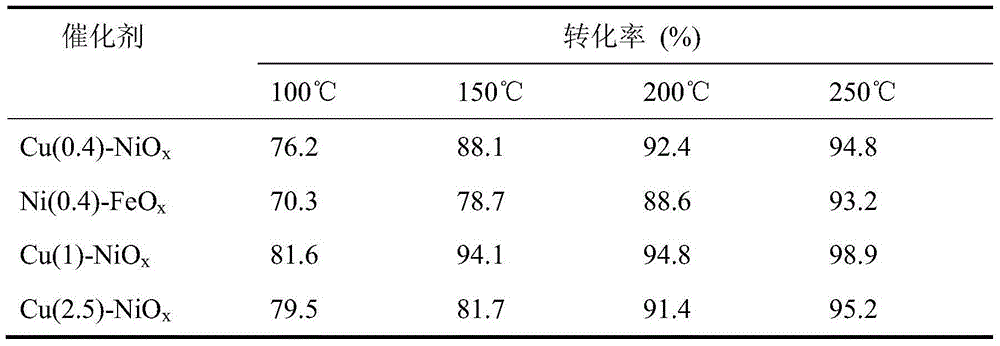
[0032] Table 2
[0033]
[0034] It can be seen from Table 2 that in the presence of oxygen, the conversion rate of NO is not affected by the oxygen concentration on various catalysts, indicating that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com