Application of chondroitin sulfate B and vaccine preparation containing same
A chondroitin sulfate and vaccine technology, applied in the field of immunology, can solve the problems of few types of vaccine adjuvants, large toxic and side effects, etc., and achieve the effects of excellent immune effect, small toxic and side effects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The dosage of the chondroitin sulfate B adjuvant provided in this example is 5 μg.
[0020] The hepatitis A vaccine containing chondroitin sulfate B adjuvant provided in this example is: add 5 μg of chondroitin sulfate B to each vaccine dose, add chondroitin sulfate B to hepatitis A (HAV) antigen, and then add normal saline to 200 μL , and mix uniformly according to routine to obtain the hepatitis A vaccine agent containing chondroitin sulfate B.
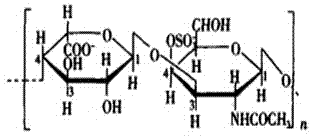
[0021] Wherein, chondroitin sulfate B is a commercially available product, and its molecular formula is (C 14 h 21 NO 14 S) n , with a molecular weight of about 41KDa, purchased from SiGMA, USA; the HAV antigen was a commercially available 18EU HAV antigen solution with a titer of 256EU / ml, purchased from the Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College.
[0022] The immune test and effect of the hepatitis A vaccine that the present embodiment 1 gained contains the cho...
Embodiment 2
[0039] The dosage of the chondroitin sulfate B adjuvant provided in this example is 65 μg.
[0040] The hepatitis A vaccine containing chondroitin sulfate B adjuvant provided in this example is: add 65 μg of chondroitin sulfate B to each vaccine dose, add chondroitin sulfate B to hepatitis A (HAV) antigen, and then add normal saline to 200 μL , and mix uniformly according to routine to obtain the hepatitis A vaccine agent containing chondroitin sulfate B. Wherein, chondroitin sulfate B and HAV antigen are the same as in Example 1;
[0041] The immune test of the hepatitis A vaccine containing the chondroitin sulfate B adjuvant obtained in this example is the same as that in Example 1, and the results are shown in Table 2.
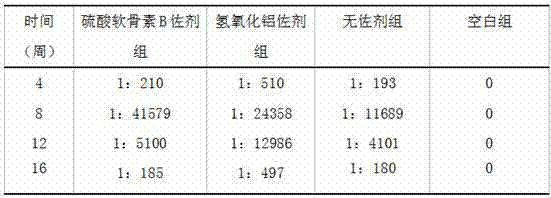
[0042] Table 2 shows the serum anti-HAV IgG antibody levels of mice in each experimental group within 16 weeks after using the adjuvant provided in Example 2.
[0043] Table 2
[0044]
[0045] It can be seen from the data analysis that except for the b...
Embodiment 3
[0047] The dosage of the chondroitin sulfate B adjuvant provided in this example is 85 μg.
[0048] The hepatitis A vaccine containing chondroitin sulfate B adjuvant provided in this example is: add 85 μg of chondroitin sulfate B to each vaccine dose, add chondroitin sulfate B to hepatitis A (HAV) antigen, and then add normal saline to 200 μL , and mix uniformly according to routine to obtain the hepatitis A vaccine agent containing chondroitin sulfate B. Wherein, chondroitin sulfate B and HAV antigen are the same as in Example 1;
[0049] The immune test of the hepatitis A vaccine containing the chondroitin sulfate B adjuvant obtained in this example is the same as in Example 1, and the results are shown in Table 3.
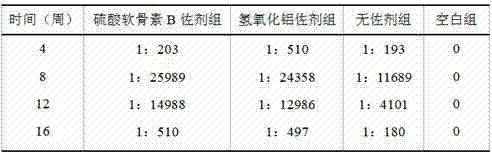
[0050] Table 3 shows the serum anti-HAV IgG antibody levels of mice in each experimental group within 16 weeks after using the adjuvant provided in Example 3.
[0051] table 3
[0052]
[0053] It can be seen from the data analysis that except for the blan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com