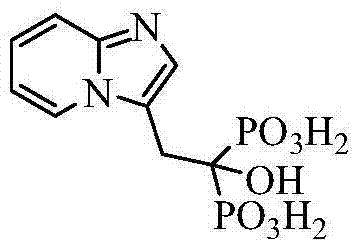
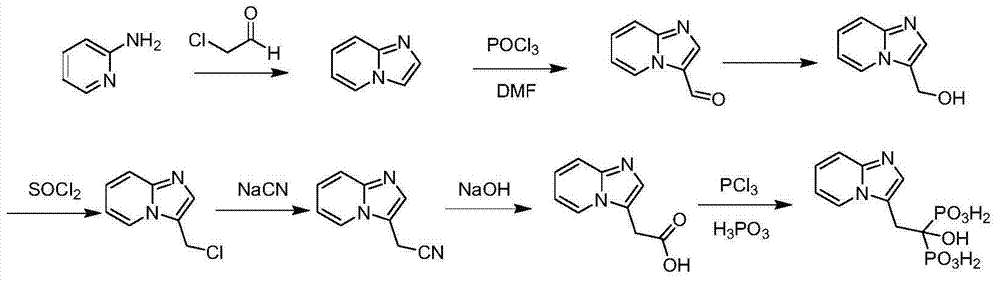
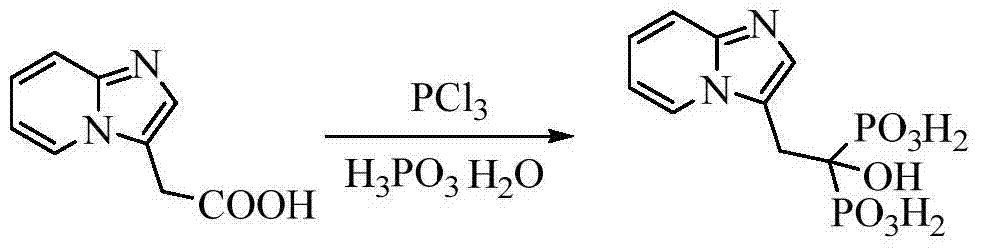Preparation method of minodronic acid
A technology of minodronic acid and phosphoric acid, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as long synthetic routes, difficult purification of products, and dangerous operation, and achieve Mild reaction conditions, short reaction steps, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1, Preparation of ethyl 4-oxobutenoate
[0042] Add 25.2g (E)-4,4-dimethoxy-2-butenoic acid ethyl ester, 40mL acetone, 20mL purified water and 2.14g p-toluenesulfonic acid into a 250ml three-necked flask; stir and heat to 40 -50°C, keep warm for 1-1.5h, concentrate the reaction solution, add 30mL of acetone, heat to 50-60°C, continue to stir for 1-2h, monitor by GC, and stop the reaction if it meets the internal control standards. The reaction solution was concentrated to obtain a yellow oily liquid.
[0043] Weigh 1.05g of sodium bicarbonate and prepare a saturated solution with 12mL of purified water for later use. Add 30mL of ethyl acetate to the above yellow liquid, cool down to 5-15°C, slowly add the pre-prepared saturated sodium bicarbonate solution, stir for 10-15min, let stand for 10-15min, separate the layers, and separate the organic layer (upper layer ); the aqueous phase was extracted once with 30mL ethyl acetate, the organic layer was separated, and the o...
Embodiment 2
[0049] 1, Preparation of ethyl 4-oxobutenoate
[0050] Add 50.4g (E)-4,4-dimethoxy-2-butenoic acid ethyl ester, 80mL acetone, 40mL purified water and 4.28g p-toluenesulfonic acid into a 500ml three-necked flask; stir and heat to 40 -50°C, keep warm for 1-1.5h, concentrate the reaction solution, add 30mL of acetone, heat to 50-60°C, continue to stir for 1-2h, monitor by GC, and stop the reaction if it meets the internal control standards. The reaction solution was concentrated to obtain a yellow oily liquid.
[0051] Weigh 2.1g of sodium bicarbonate and prepare a saturated solution with 24mL of purified water for later use. Add 60mL of ethyl acetate to the above yellow liquid, cool down to 5-15°C, slowly add the pre-prepared saturated sodium bicarbonate solution, stir for 10-15min, let stand for 10-15min, separate the layers, and separate the organic layer (upper layer ); the aqueous phase was extracted once with 60mL ethyl acetate, the organic layer was separated, and the or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com