Method for synthesizing 3,4,5-trifluoro-2'-nitrobiphenyl
A technology of nitrobiphenyl and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, molecular sieve catalysts, etc., can solve problems such as catalyst problems, lack of good quality, and difficulty in large-scale application, and achieves short synthesis steps. , No three wastes discharge, simple treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
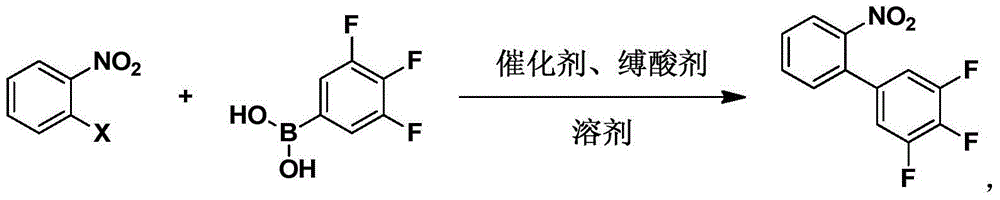
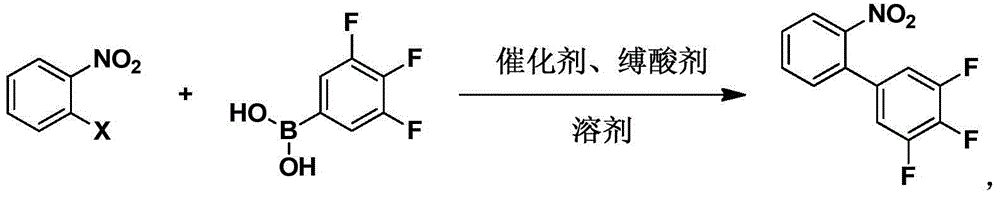
[0042] The present invention also provides a method for synthesizing 3,4,5-trifluoro-2'-nitrobiphenyl, which is characterized in that it comprises:
[0043] Step 1: Dissolve raw materials 3,4,5-trifluorophenylboronic acid and o-nitro-substituted benzene in a solvent, and add a catalyst and an acid binding agent;
[0044] Step 2: Stir the reaction to the end;
[0045] Step 3: Wash with water and extract the organic phase;
[0046] Step 4: Concentrate and recrystallize to obtain 3,4,5-trifluoro-2'-nitrobiphenyl.
[0047] According to a preferred embodiment of the second aspect of the present invention, in step 1:
[0048] Wherein, the solvent is selected from any one of protic solvents and aprotic solvents: the protic solvents include methanol, ethanol, propanol, isopropanol, water, formic acid, and acetic acid, preferably methanol; Aprotic solvents include N,N-dimethylformamide, acetone, ethyl acetate, dichloromethane, ether, carbon tetrachloride, toluene, benzene, n-hexane, cyclohexane,...
Embodiment 1
[0069] Example 1-About the screening of catalysts
[0070] 1. Synthetic route:
[0071]
[0072] 2. Synthesis method (according to Table 1):
[0073] Step 1: Set up 4 reactions, dissolve raw materials 3,4,5-trifluorophenylboronic acid and o-chloronitrobenzene in solvent DMF, add acid binding agent triethylamine, and add catalyst PtCl respectively 2 , Pd(OAc) 2 , Pd(PPh 3 ) 4 , Ms-Pd, the addition amount is 0.5%;
[0074] Step 2: Stir the reaction at 30°C, and stop the reaction after the liquid phase tracking raw material reaction is completed;
[0075] Step 3: Recover the solvent and catalyst, and the crude product is washed and extracted to obtain an organic phase;
[0076] Step 4: Concentrate and recrystallize from n-hexane to obtain 3,4,5-trifluoro-2'-nitrobiphenyl. The best result is 24.0g, light yellow crystalline solid, melting point 78.1-78.3℃, liquid content 97% . 1 H NMR(400MHz, CDCl 3 )δ8.00-8.05(m,2H),7.90-7.75(m,1H),7.67-7.55(m,1H),7.27-7.14(m,2H)ppm; 13C NMR(100MHz,CDCl3)δ1...
Embodiment 2
[0080] Example 2-Screening on -X
[0081] 1. Synthetic route:
[0082]
[0083] 2. Synthesis method (according to Table 2):
[0084] Step 1: Open 3 reactions, respectively dissolve raw materials 3,4,5-trifluorophenylboronic acid and 3 kinds of o-nitro-substituted benzene in solvent methanol, and add acid binding agent sodium bicarbonate and catalyst PdCl 2 , The addition amount is 0.5%;
[0085] Step 2: Stir the reaction at 40°C, and stop the reaction after the liquid phase tracking raw material reaction is completed;
[0086] Step 3: Recover the solvent and catalyst, and the crude product is washed and extracted to obtain an organic phase;
[0087] Step 4: Concentrate to obtain 3,4,5-trifluoro-2'-nitrobiphenyl.
[0088] 3. Results:
[0089] Table 2: Test results of -X screening
[0090] Numbering
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com