Preparation method and application of arctigenin derivatives
A technology of arctigenin and its derivatives, which is applied in the field of preparation of arctigenin derivatives, can solve the problems of arctigenin anti-cancer new drug development difficulties, low bioavailability, and low water solubility, and achieve solvent-saving and preparation The effect of simple process and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
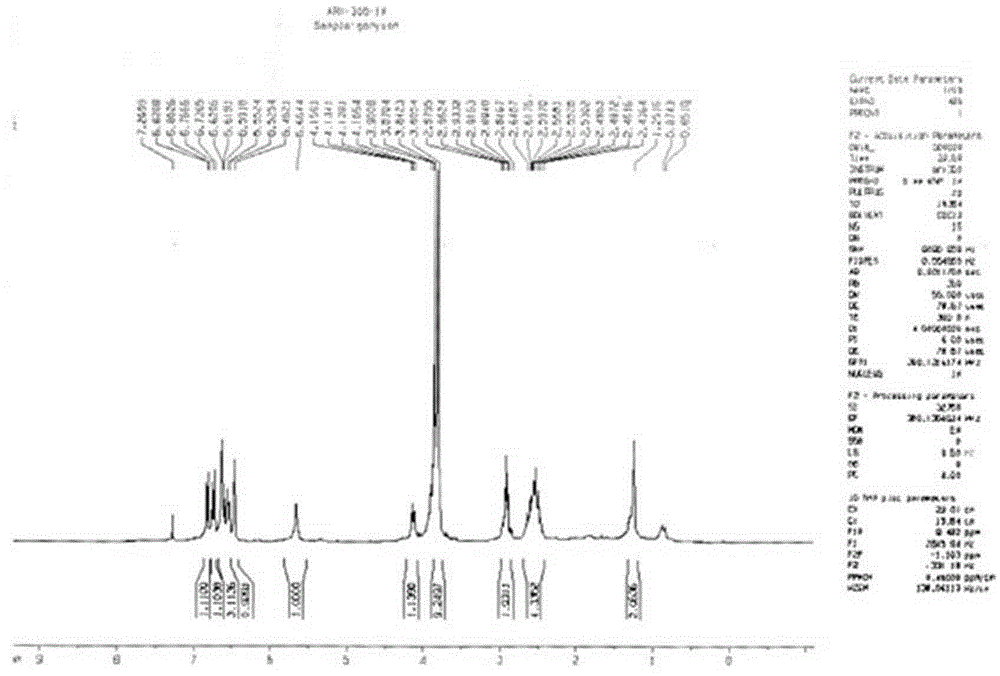
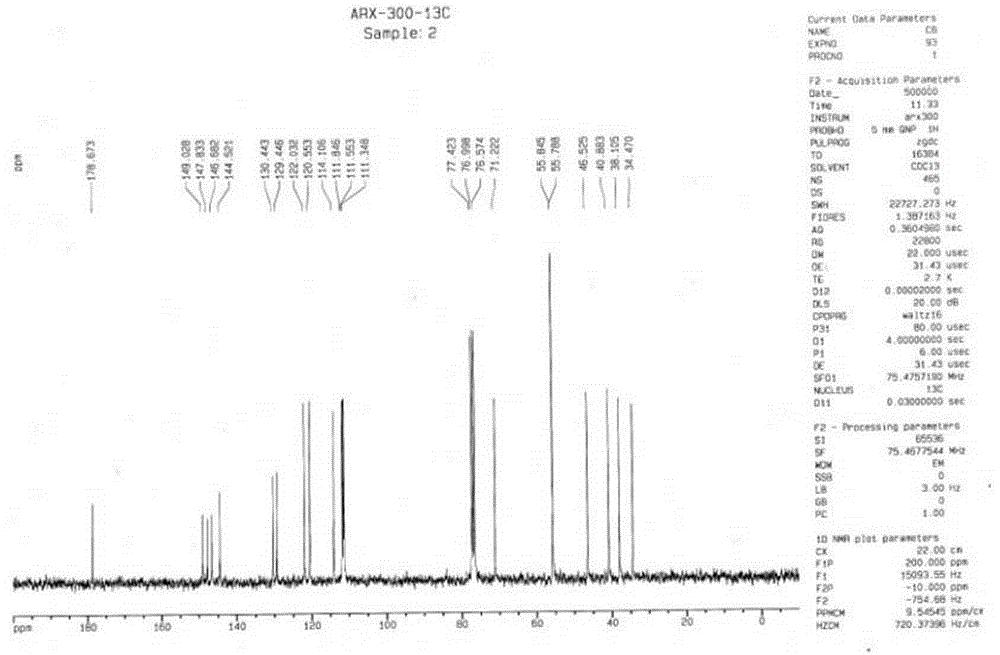
[0029] Add 74.8 mg arctigenin and 2 mL ethanolamine solution into a round bottom flask, stir at room temperature, and stop the reaction. The reaction liquid is concentrated under reduced pressure, and the residual liquid is separated by high-efficiency preparation liquid phase to obtain the ammonolysis derivative of arctigenin 1, Among them compounds 1 for new compounds.
[0030]
[0031] Measuring conditions: INSTROM AV500
[0032] Solvent: CDCl 3
[0033] Table 1 Compound 1 13 C-NMR and 1 H-NMR nuclear magnetic resonance data
[0034]
preparation example 2
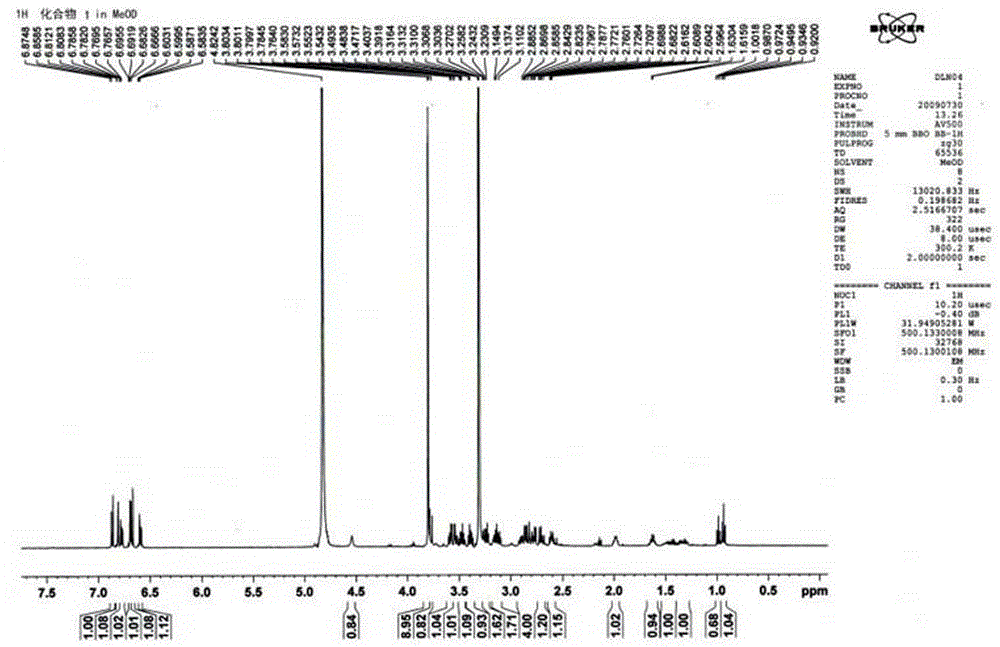
[0036] 111.6mg of arctigenin was placed in a 25mL round bottom flask, 6.0mL of n-propylamine solution was added, stirred and dissolved at 25°C, and the reaction was stopped after 24 hours of reaction. The reaction solution was concentrated under reduced pressure and recrystallized from methanol to obtain the compound 2 , where the compound 2 for new compounds.
[0037]
[0038] Measuring conditions: INSTROM AV500
[0039] Solvent: CDCl 3
[0040] Table 2 Compound 2 13 C-NMR and 1 H-NMR nuclear magnetic resonance data
[0041]
preparation example 3
[0043] Add 96mg arctigenin and 1mL benzylamine solution into a round bottom flask, and react at 160°C for 12 hours. Cool the reaction solution to room temperature, add 10% dilute hydrochloric acid to the reaction solution, extract 3-5 times with ethyl acetate, combine the extracts, concentrate, and then use high-efficiency preparative liquid phase for separation to obtain the compound 3 .
[0044]
[0045] Measuring conditions: INSTROM AV500
[0046] Solvent: CDCl 3
[0047] Table 3 Compound 3 13 C-NMR and 1 H-NMR nuclear magnetic resonance data
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com