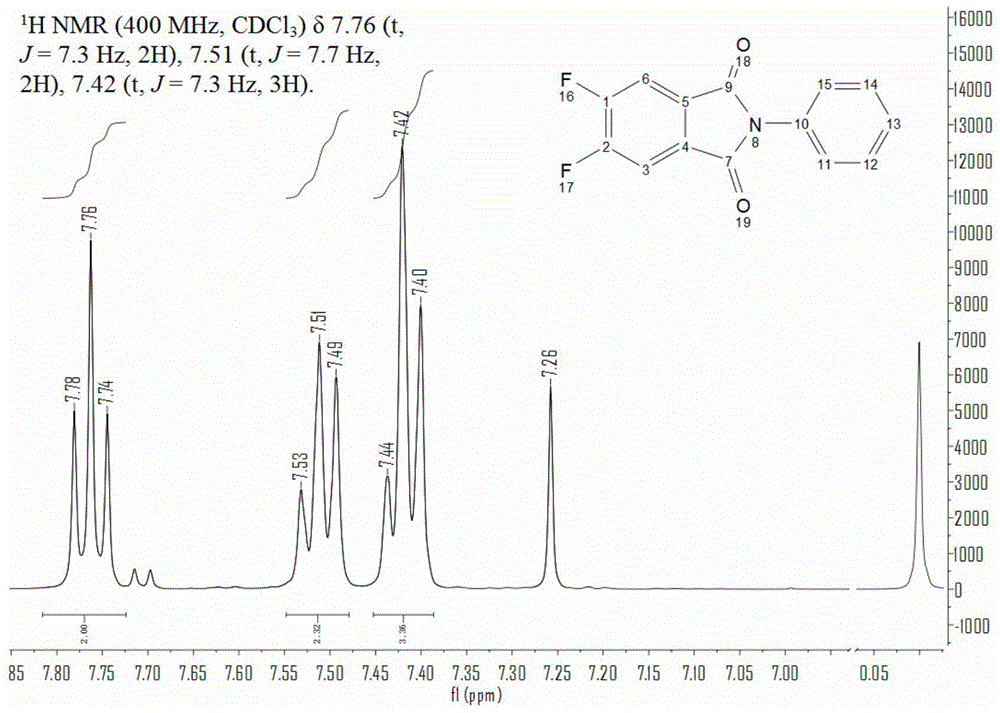
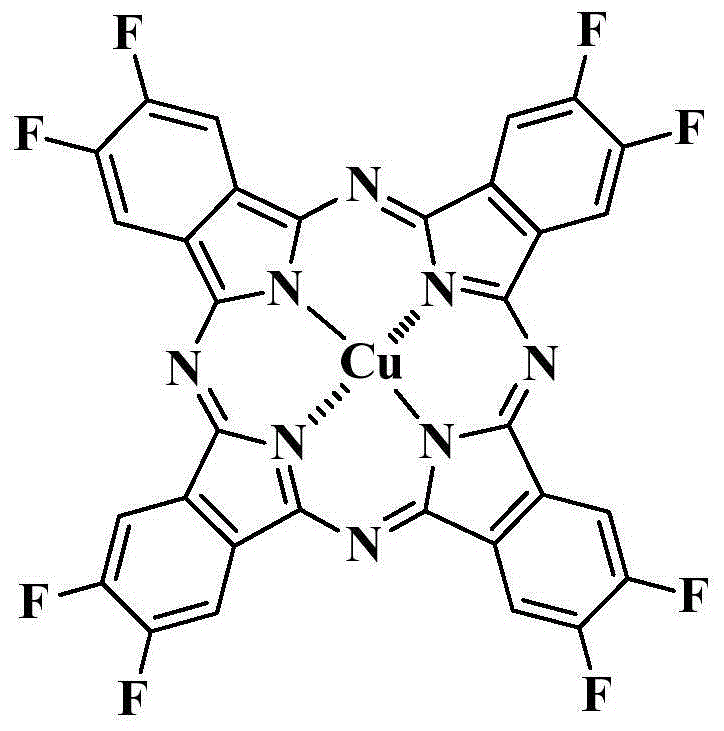
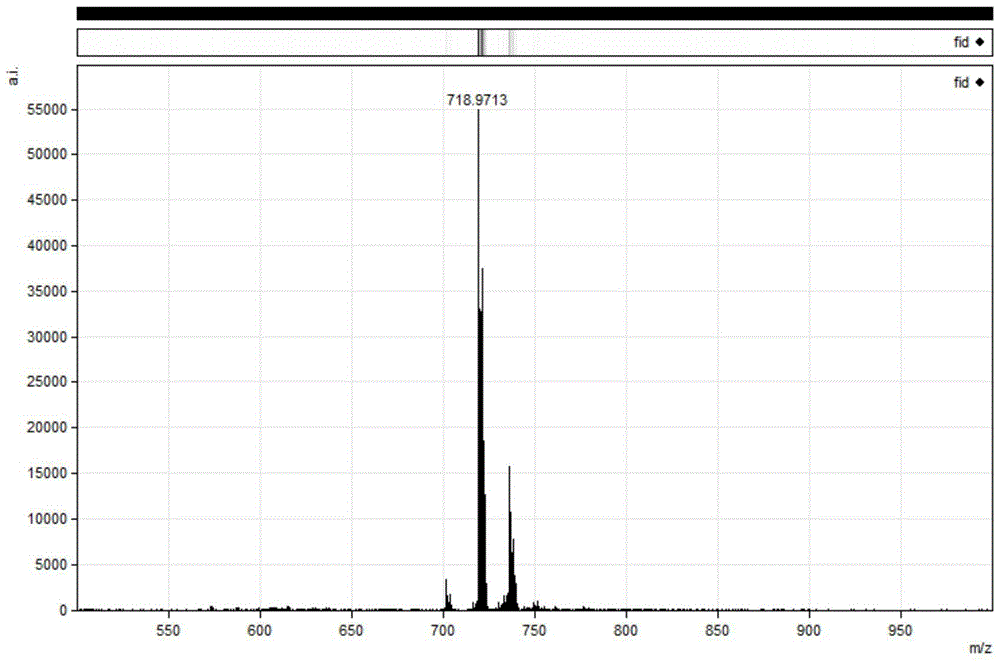
The preparation method of octafluoro substituted phthalocyanine
A technology for octafluoro-substituted phthalocyanines, which is applied in the field of preparation of octafluoro-substituted phthalocyanines, can solve the problems of few synthesis methods of octafluoro-substituted phthalocyanines, and achieve the effects of low raw material cost, high yield, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036](1) Preparation of 4,5-dichlorophthalic anhydride: weigh 4,5-dichlorophthalic acid (10.15g, 43mmol) into a 50ml four-necked flask, then add acetic anhydride (30ml, 294mmol), Stir magnetically, heat, and raise the temperature to 110°C to dissolve all the solids, continue to heat up to 140°C, react for 1 hour, spot plate detection, the raw materials are completely reacted, stop the reaction. After cooling and standing to room temperature, a large amount of white flaky crystals precipitated, filtered, the filter cake was washed three times with petroleum ether, and vacuum-dried to obtain 9.69 g of white flaky crystals and white powder solids. The purity measured by high performance liquid chromatography was 99.9%. Efficiency: 95.09% (literature value 93%), melting point: 185-187°C (literature value 185-187°C).
[0037] (2) Synthesis of N-phenyl-4,5-dichlorophthalimide: Take 4,5-dichlorophthalic anhydride (5g, 0.023mol), aniline 2.80ml, anhydrous ice Add 40ml of acetic acid...
Embodiment 2
[0046] (1) Preparation of 4,5-dichlorophthalic anhydride: Add acetic anhydride (13ml, 125mmol) and react for 2.5h to obtain 8.90g of white flaky crystals and white powder. Other conditions and parameters are the same as those in the first embodiment.
[0047] (2) Synthesis of N-phenyl-4,5-dichlorophthalimide: take 4,5-dichlorophthalic anhydride (5g, 0.023mol), aniline 9.0ml, anhydrous ice Add 50ml of acetic acid into a 100ml four-necked flask in turn, stir magnetically, heat to reflux to 100°C, and react for 5h to obtain 5.32g of the product. Other conditions and parameters are the same as those in the first embodiment.
[0048] (3) Synthesis of N-phenyl-4,5-difluorophthalimide: put dry sodium fluoride (1.47g, 35mmol) into a dry four-neck flask, add redistilled N, N-dimethylformamide 10ml, magnetic stirring, heating up to 150°C, then adding dry N-phenyl-4,5-dichlorophthalimide (1.00g, 3.4mmol) in sequence, phase transfer Catalyst tetrabutylammonium bromide (0.02g, 0.06mmol)...
Embodiment 3
[0052] (1) Preparation of 4,5-dichlorophthalic anhydride: add acetic anhydride (85ml, 125mmol) and react for 4h to obtain 9.12g of white flaky crystals and white powder. Other conditions and parameters are the same as those in the first embodiment.
[0053] (2) Synthesis of N-phenyl-4,5-dichlorophthalimide: Take 4,5-dichlorophthalic anhydride (5g, 0.023mol), distill 20ml of aniline newly, Add 40ml of water and glacial acetic acid to a 100ml four-necked flask in sequence, stir magnetically, heat to 120°C, react for 10h, stop the reaction, and obtain 4.89g of the product. Other conditions and parameters are the same as those in the first embodiment.
[0054] (3) Synthesis of N-phenyl-4,5-difluorophthalimide: put dry cesium fluoride (2.97g, 30mmol) into a four-neck flask, add 20ml of dimethyl sulfoxide , magnetically stirred, heated up to 150°C, then added dry N-phenyl-4,5-dichlorophthalimide (2.00g, 7mmol), phase transfer catalyst polyethylene glycol-6000 (0.12 g, 0.02mmol) w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com