1-ferrocenyl-3-aryl-3-(2,6-dicarbonyl-4-thiopyrimidinyl)-acetone and preparation method thereof
A thiopyrimidine-based, ferrocene-based technology, applied in chemical instruments and methods, metallocenes, chemicals for biological control, etc., can solve the problems of large solvent usage, long reaction time, low yield, etc. , to achieve the effect of short reaction time, simple reaction process and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Weigh 0.0012mol thiobarbituric acid, 0.0012mol anhydrous K 2 CO 3 Put it in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-phenylpropenone, mix and grind. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with water, filter, and dry to obtain a dark red solid, which is 1-ferrocene Base-3-phenyl-3-(2,6-dicarbonyl-4-thiopyrimidinyl)-propanone. Yield 75%, m.p. 127°C-129°C.
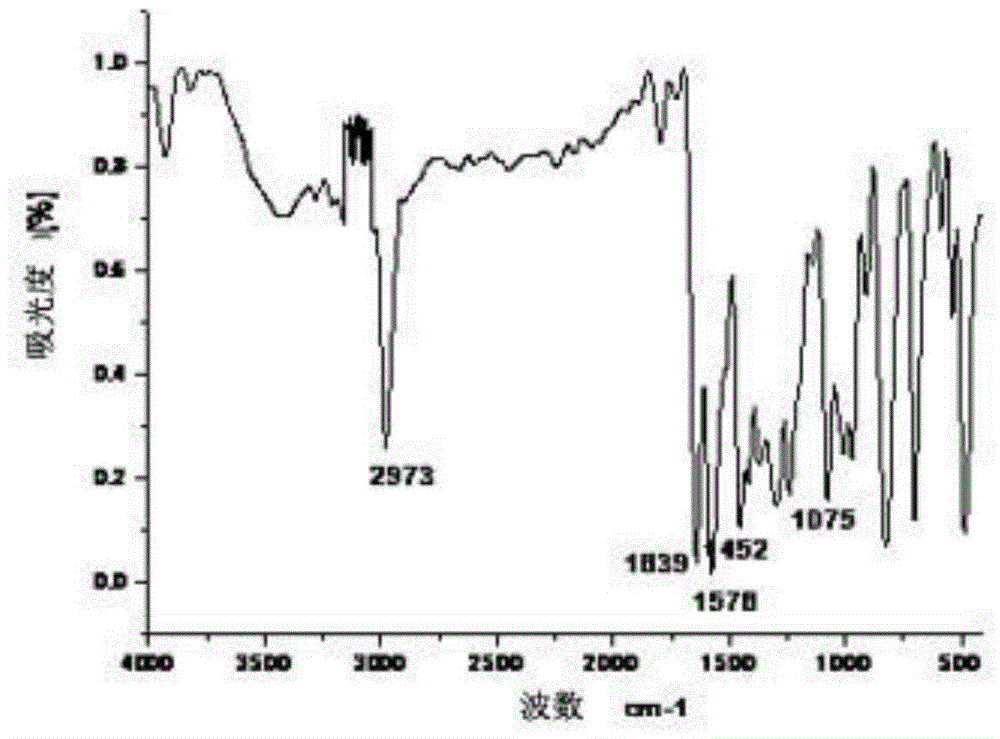
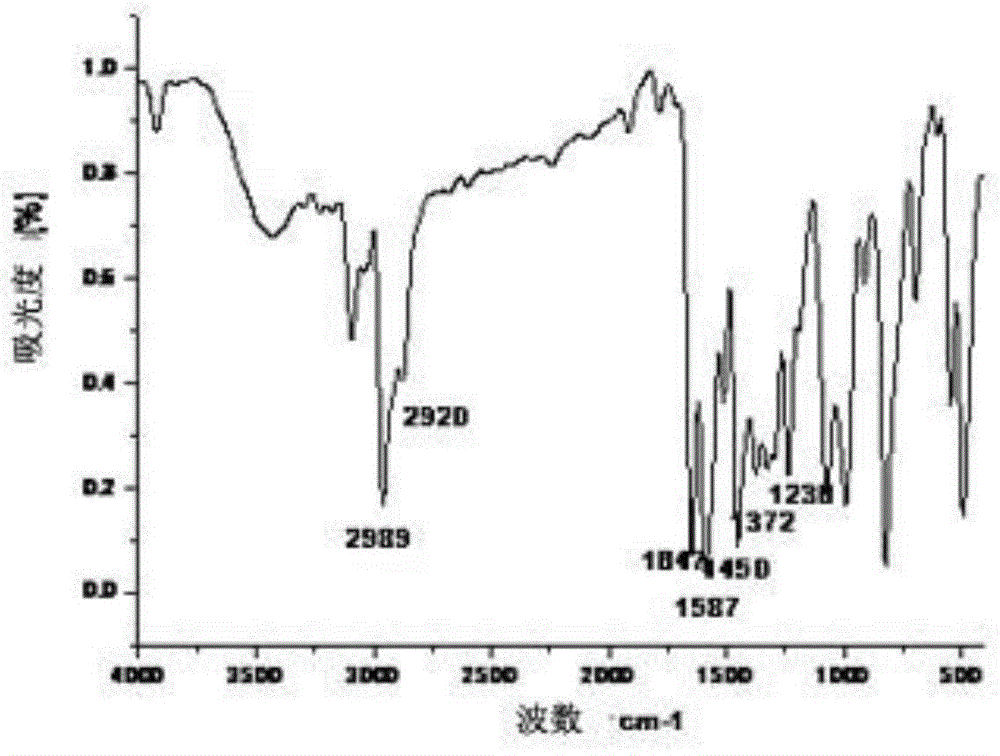
[0059] IR (KBr tablet, v / cm -1 ):3440, 2921, 1782, 1715, 1646, 1595, 1494, 1450, 1073;
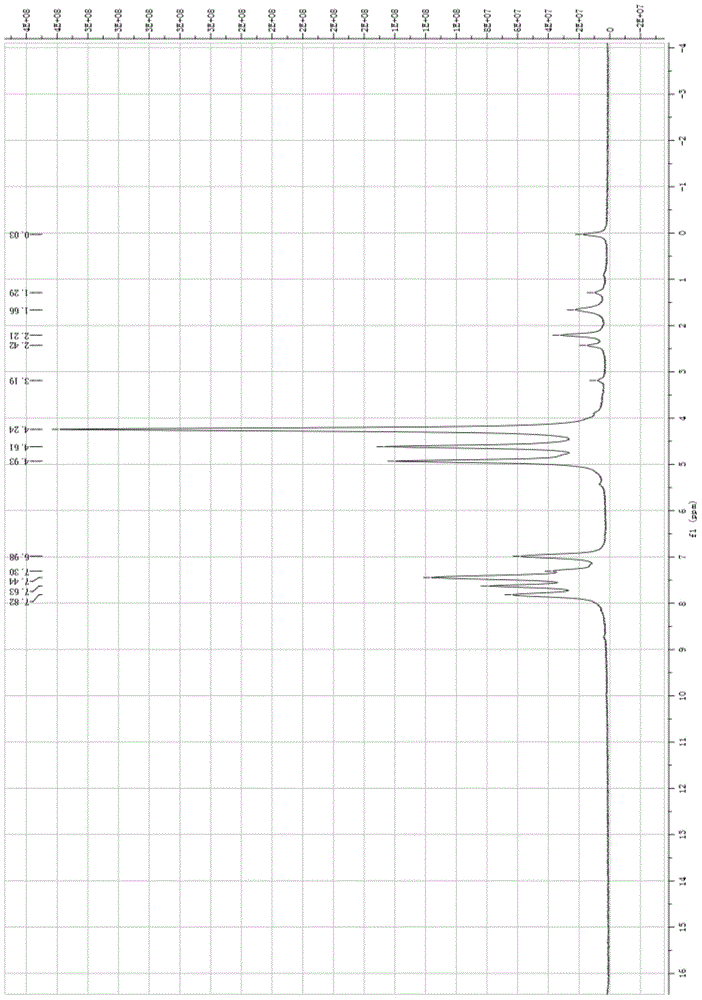
[0060] 1 H-NMR: 7.14-7.85 (m, 5H, Ar-H), 6.70 (s, 1H, -CONH), 4.95 (s, 2H, C 5 h 4 ), 4.63 (s, 2H, C 5 h 4 ), 4.25(s, 5H, C 5 h 5 ), 2.03(d,2H,-COCH 2 ), 1.70(m, 1H, -CH);
[0061] 13 C-NMR: 192.5, 140.4, 134.7, 128.5, 127.8, 122.5, 43.1, 28.9.
Embodiment 2
[0063] Weigh 0.0012mol thiobarbituric acid, 0.0012mol anhydrous K 2 CO 3 Put it in a mortar and mix quickly and evenly, then add 0.001mol 1-ferrocenyl-3-(p-chlorophenyl)-propenone, mix and grind. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with water, filter, and dry to obtain a dark red solid, which is 1-ferrocene Base-3-(p-chlorophenyl)-3-(2,6-dicarbonyl-4-thiopyrimidinyl)-propanone. Yield 76%, m.p. 150°C-151°C.
[0064] IR (KBr tablet, v / cm -1 ):3420, 2920, 1783, 1715, 1646, 1598, 1450, 1077;
[0065] 1 H-NMR: 7.12-7.77 (m, 4H, Ar-H), 6.72 (s, 1H, -CONH), 4.94 (s, 2H, C5H 4 ), 4.64(s, 2H, C 5 h 4 ), 4.25(s, 5H, C 5 h 5 ), 2.05(d,2H,-COCH 2 ), 1.65(m, 1H, -CH);
[0066] 13 C-NMR: 192.8, 138.9, 135.7, 134.2, 128.9, 122.9, 43.8, 30.2.
Embodiment 3
[0068] Weigh 0.0012 mol of thiobarbituric acid and 0.0012 mol of NaOH in a mortar and mix quickly and uniformly, then add 0.001 mol of 1-ferrocenyl-3-(p-bromophenyl)-propenone, mix and grind. The mixture will become viscous as the reaction proceeds. Continue to grind until the substance does not change. Use thin-layer chromatography to monitor the reaction progress. After the reaction is completed, wash with water, filter, and dry to obtain a dark red solid, which is 1-ferrocene Base-3-(p-bromophenyl)-3-(2,6-dicarbonyl-4-thiopyrimidinyl)-propanone. Yield 73%, m.p. 159°C-160°C.
[0069] IR (KBr tablet, v / cm -1 ):3437, 2925, 1788, 1712, 1648, 1589, 1450, 1069;
[0070] 1 H-NMR: 7.16-7.57 (m, 4H, Ar-H), 6.71 (s, 1H, -CONH), 4.94 (s, 2H, C 5 h 4 ), 4.63 (s, 2H, C 5 h 4 ), 4.24(s, 5H, C 5 H5), 2.02(d,2H,-COCH 2 ), 1.65(m, 1H, -CH);
[0071] 13 C-NMR: 192.8, 139.7, 136.5, 131.7, 129.2, 122.8, 43.9, 31.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com