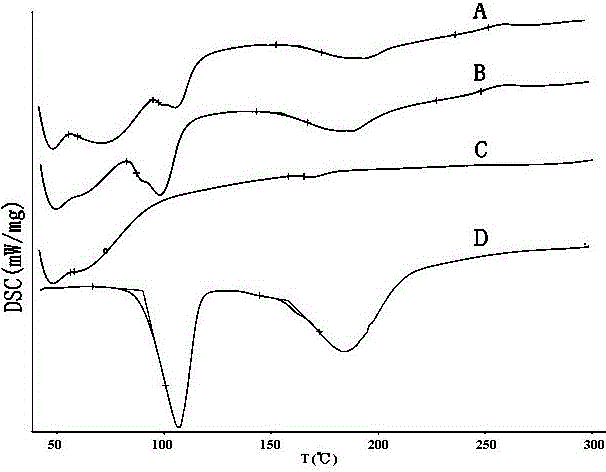
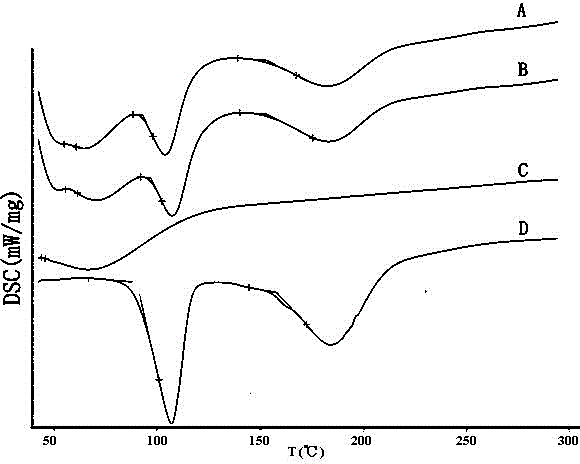
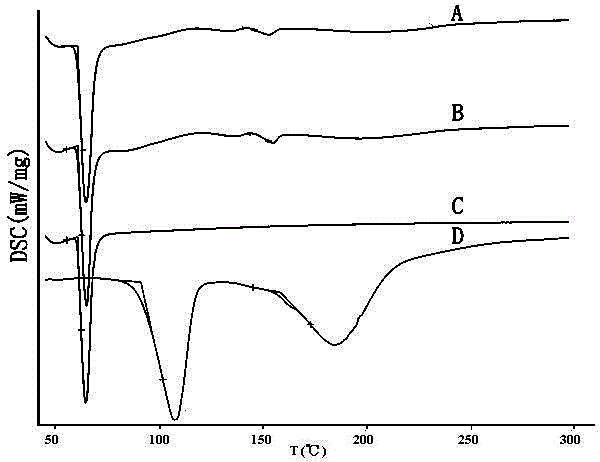
Mosapride citrate co-grinded product, preparation method thereof and medicine composition containing mosapride citrate co-grinded product
A co-grinding technology of mosapride citrate, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of low mosapride citrate content, low bioavailability, low dissolution rate, etc. , to achieve the effect of improving bioavailability and clinical treatment effect, overcoming cumbersome preparation methods, good repeatability and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Mix 5.31 g of mosapride citrate and 20.21 g of HPMC, put them in a PM-100 ball mill for co-grinding for 1 hour, take them out, sieve them, and store them in a silica gel desiccator away from light. The co-grinding product was tested for dissolution, and the results are shown in Table 5.
Embodiment 2
[0063] Mix 5.31 g of mosapride citrate and 6.06 g of HPMC evenly, put them in a PM-100 ball mill and grind them together for 1 hour, take them out, sieve them, and store them in a silica gel desiccator away from light. Dissolution test was carried out on this co-grinding material, and the results are shown in Table 5.
Embodiment 3
[0073] Mix 5.32 g of mosapride citrate and 20.20 g of L-HPC evenly, grind in a PM-100 ball mill for 1 hour, take out, sieve, and store in a silica gel desiccator away from light. Dissolution test was carried out on this co-grinding material, and the results are shown in Table 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com