Tablet and preparation method thereof
A tablet and adhesive technology, which is applied in the direction of pill delivery, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problems of unsatisfactory tablet appearance, difficult process stability, and tablet stability, etc. problems, to achieve the effect of bright surface, improved appearance and good molding quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
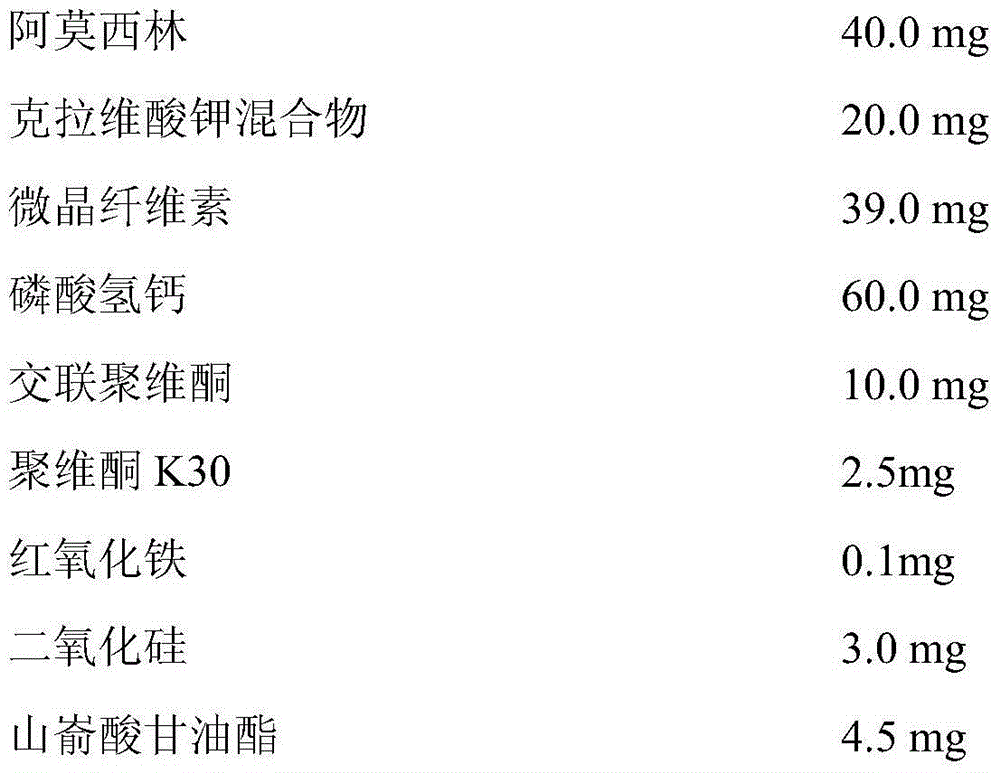
[0029] When the compound amoxicillin-clavulanate potassium tablet of the present embodiment is compressed, the quality of each tablet is controlled at 180 ± 13.5mg, and each tablet is configured according to the following components and dosage:
[0030]
[0031]
[0032] The potassium clavulanate mixture is a mixture formed by mixing potassium clavulanate and microcrystalline cellulose according to a mass ratio of 1:1. Cross-linked povidone, red iron oxide and absolute alcohol are configured as binders and added during tablet preparation, and absolute alcohol volatilizes during tablet preparation, leaving no residue in the finished tablet.
[0033] The preparation method of the compound recipe amoxicillin-clavulanate potassium tablet of the present embodiment comprises the following specific steps:
[0034] A. Ingredients: Weigh an appropriate amount of amoxicillin powder, potassium clavulanate mixture, microcrystalline cellulose, calcium hydrogen phosphate, crospovidone...
Embodiment 2
[0041] The remainder of the compound recipe amoxicillin-clavulanate potassium tablet of the present embodiment is identical with embodiment 1, and difference is:
[0042] The quality of each tablet is controlled at 900±45mg during tablet compression, and each tablet is configured according to the following components and dosage:
[0043]
[0044]
[0045] The rest of the preparation method of the compound amoxicillin-clavulanate potassium tablet of the present embodiment is the same as that of Example 1, except that in step B, the prepared amoxicillin powder, microcrystalline cellulose, phosphoric acid Calcium hydrogen and crospovidone were added to the granulator and mixed for 8 minutes. In step C, the prepared amoxicillin granules and the prepared mixture of potassium clavulanate, silicon dioxide, glyceryl behenate and beef flavor essence are added into a three-dimensional motion mixer, mixed for 12 minutes, and then discharged. In step D, the raw material is compress...
Embodiment 3
[0047] The remainder of the compound recipe amoxicillin-clavulanate potassium tablet of the present embodiment is identical with embodiment 1, and difference is:
[0048] The quality of each tablet is controlled at 1800±36mg during tablet compression, and each tablet is configured according to the following components and dosage:
[0049]
[0050] The rest of the preparation method of the compound amoxicillin-clavulanate potassium tablet of the present embodiment is the same as that of Example 1, except that in step B, the prepared amoxicillin powder, microcrystalline cellulose, phosphoric acid Calcium hydrogen and crospovidone were added to the granulator and mixed for 3 minutes. In step C, the prepared amoxicillin granules and the prepared mixture of potassium clavulanate, silicon dioxide, glyceryl behenate and beef flavor essence are added into a three-dimensional motion mixer, mixed for 8 minutes, and then discharged. In step D, the raw material is compressed into a ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com