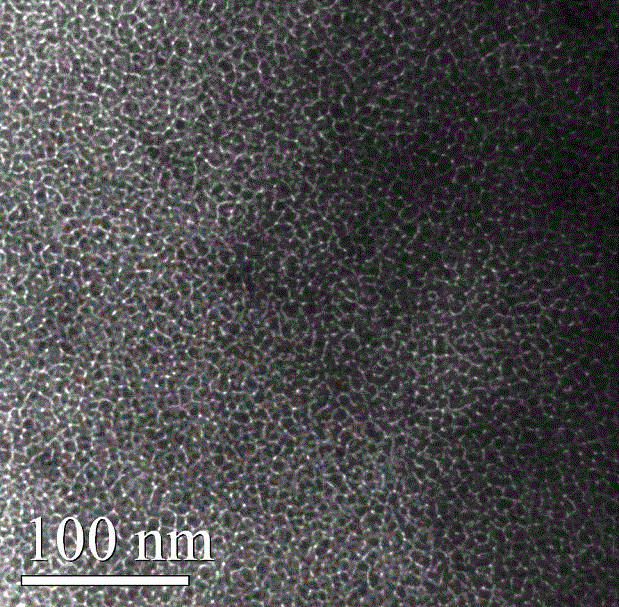
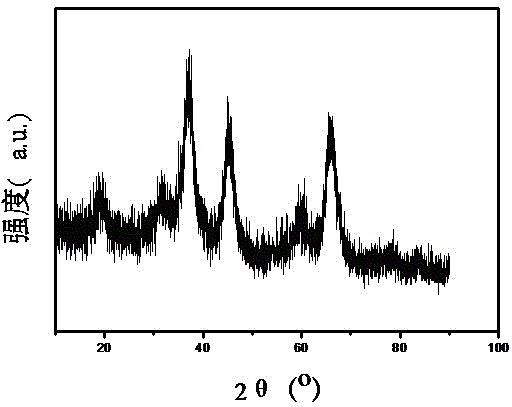
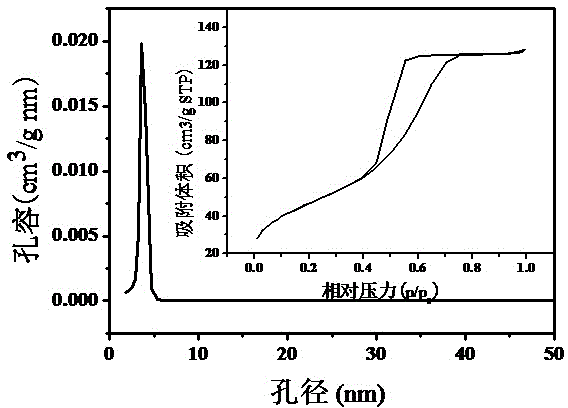
Mesoporous alumina nickel-based catalyst with high activity and high stability for CO2 reforming CH4 reaction and preparation method of mesoporous alumina nickel-based catalyst
A catalyst and mesoporous technology, applied in the field of mesoporous aluminum-nickel-based catalysts and their preparation, can solve problems such as sulfide poisoning, expensive noble metal catalysts, harsh operating conditions, etc., and achieve large pore volume, high catalytic activity and stability , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation steps in the present embodiment are as follows:
[0041] a. Add 3.9 g Ni(NO 3 ) 2 .6H 2 O and 37.5 g Al(NO 3 ) 3 .9H 2 O was dissolved in 50 mL of deionized water to obtain a uniformly mixed solution so that the concentration of aluminum ions was 2 mol / L;
[0042] b. Under the condition of 75 ℃, add ammonium carbonate solution with a concentration of 1 mol / L to the above solution drop by drop while stirring, control the titration rate of ammonium carbonate to 0.4 mL / min, and stop immediately after the sol is obtained through the titration reaction Titration, control the pH value of the titrated sol to be 5.0~5.5;
[0043] c. Aging the sol formed by titration at room temperature for 24 hours at a constant temperature, spread it in an open container with a thickness of no more than two centimeters, and dry it at a constant temperature of 100°C for 24 hours;
[0044] d. Calcining the above-mentioned dried gel at 200 °C for 10 h at a heating rate of...
Embodiment 2
[0048] The preparation steps in the present embodiment are as follows:
[0049] a. Add 4.1g Ni(NO 3 ) 2 ·6H 2 O, 0.6g La(NO 3 ) 3 ·nH 2 O and 37.5g Al(NO 3 ) 3 9H 2 O was dissolved in 50mL of deionized water to obtain a uniformly mixed solution so that the concentration of aluminum ions was 2mol / L;
[0050] b. Under the condition of 75 ℃, add ammonium carbonate solution with a concentration of 1mol / L to the above solution drop by drop while stirring, and control the titration rate of ammonium carbonate to about 0.4 mL / min, so that after the titration reaction to obtain the sol Stop the titration immediately, and control the pH value of the titrated sol to be 5.0~5.5;
[0051] c. Aging the sol formed by titration at room temperature for 24 hours at a constant temperature, spread it in an open container with a thickness of no more than two centimeters, and dry it at a constant temperature of 100°C for 24 hours;
[0052] d. Calcining the above-mentioned dried gel at 2...
Embodiment 3
[0055] The preparation steps in the present embodiment are as follows:
[0056] a. Add 4.1g Ni(NO 3 ) 2 ·6H 2 O, 0.6g Ce(NO 3 ) 3 ·6H 2 O and 37.5g Al(NO 3 ) 3 9H 2 O was dissolved in 50mL of deionized water to obtain a uniformly mixed solution so that the concentration of aluminum ions was 2mol / L;
[0057] b. Under the condition of 75 ℃, add ammonium carbonate solution with a concentration of 1mol / L to the above solution dropwise while stirring, and control the titration rate of ammonium carbonate to about 0.4mL / min, so that after the titration reaction to obtain the sol Stop the titration immediately, and control the pH value of the titrated sol to be 5.0~5.5;
[0058] c. Aging the sol formed by titration at room temperature for 24 hours at a constant temperature, spread it in an open container with a thickness of no more than two centimeters, and dry it at a constant temperature at 100°C for 24 hours;
[0059] d. Calcining the above-mentioned dried gel at 200 °C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com