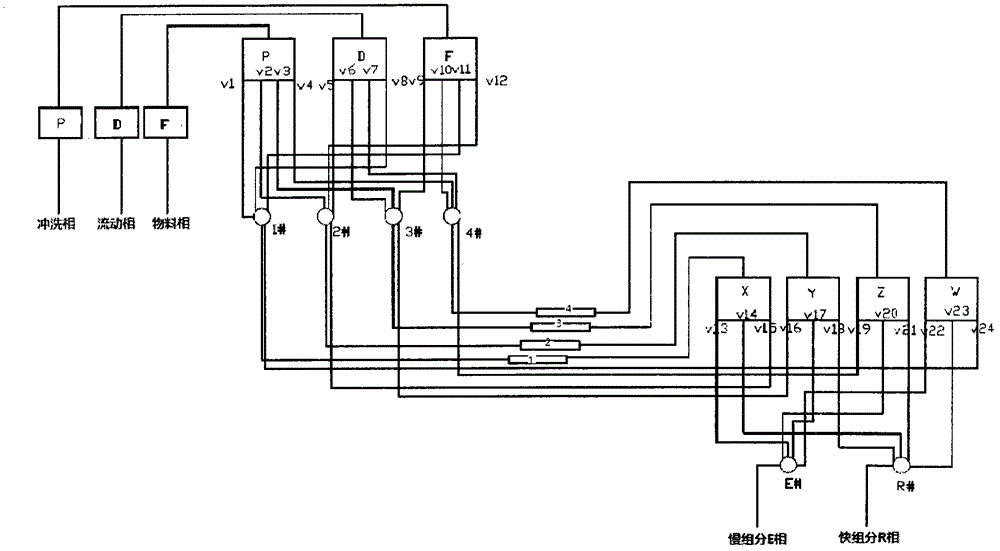
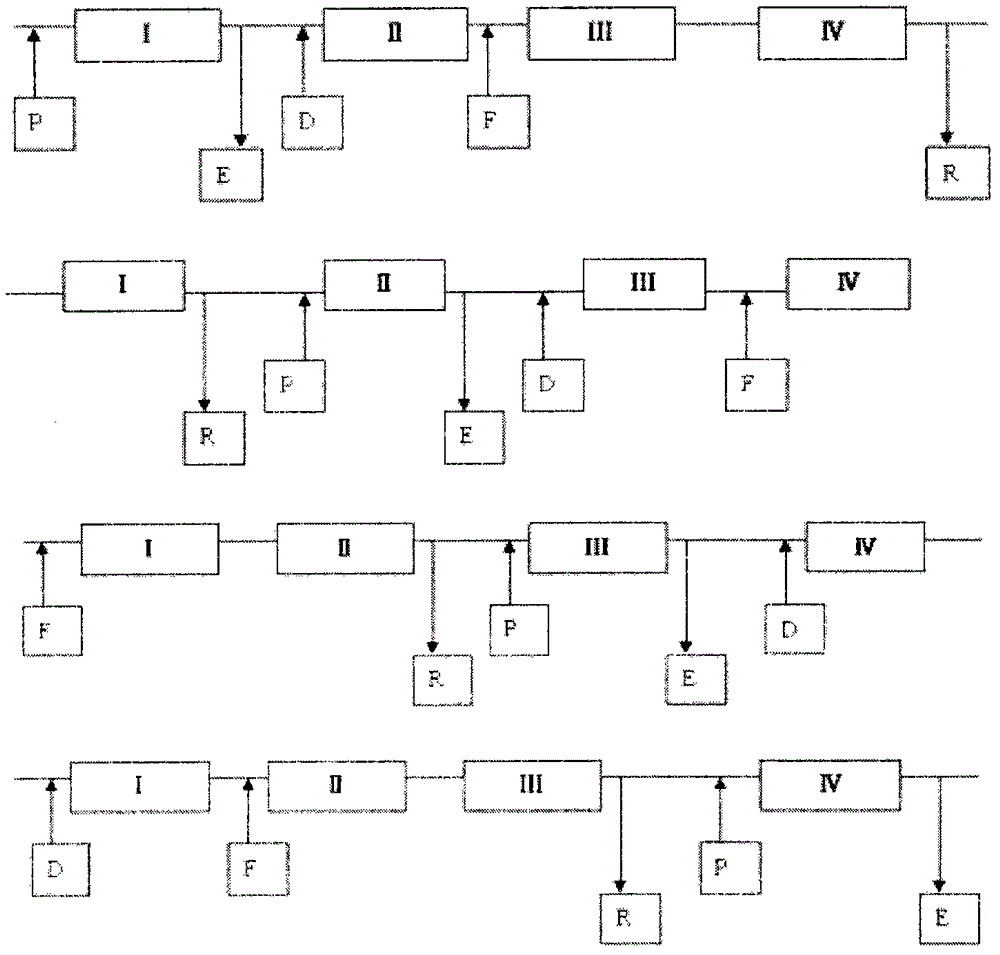
Method for preparing donepezil by virtue of simulated moving bed chromatographic separation
A technology for simulating moving bed and donepezil, which is applied to the field of separation and preparation of donepezil by simulated moving bed chromatography, can solve the problems of ineffective utilization and no technical method for separating donepezil enantiomers, etc., and achieves the effects of stable and reliable product and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example example 1
[0035] operating conditions
[0036] Mobile phase: absolute ethanol
[0037] Flow rate: 1ml / min
[0038] Injection concentration: Donepezil racemate: 0.2mg / ml
[0039] Injection liquid flow rate: v F =0.1ml / min
[0040] Mobile phase flow rate: v D =1.0ml / min
[0041] Flushing fluid flow rate: v P =2.0ml / min
[0042] F pump pressure: 6.8MPa
[0043] P pump pressure: 7.5MPa
[0044] D pump pressure: 7.1MPa
[0045] Switching time: 450s
[0046] Column temperature: 25°C
[0047] Finished product analysis
[0048] The composition of extract and raffinate was analyzed by chiral column Chiralcel-OJ. The content of L-donepezil or D-donepezil is 100%, 95.5%.
specific Embodiment 2
[0049] operating conditions
[0050] Mobile phase: absolute ethanol
[0051] Flow rate: 1ml / min
[0052] Injection concentration: Donepezil racemate: 0.2mg / ml
[0053] Injection liquid flow rate: v F =0.1ml / min
[0054] Mobile phase flow rate: v D =1.0ml / min
[0055] Flushing fluid flow rate: v P =2.0ml / min
[0056] F pump pressure: 6.2MPa
[0057] P pump pressure: 8.1MPa
[0058] D pump pressure: 6.7MPa
[0059] Switching time: 480s
[0060] Column temperature: 25°C
[0061] Finished product analysis
[0062] The composition of extract and raffinate was analyzed by chiral column Chiralcel-OJ. The content of L-donepezil or D-donepezil is 100%, 84.5%.
specific Embodiment 3
[0063] operating conditions
[0064] Mobile phase: absolute ethanol
[0065] Flow rate: 1ml / min
[0066] Injection concentration: Donepezil racemate: 0.2mg / ml
[0067] Injection liquid flow rate: v F =0.1ml / min
[0068] Mobile phase flow rate: v D =1.0ml / min
[0069] Flushing fluid flow rate: v P =2.0ml / min
[0070] F pump pressure: 6.6MPa
[0071] P pump pressure: 7.5MPa
[0072] D pump pressure: 6.8MPa
[0073] Switching time: 510s
[0074] Column temperature: 25°C
[0075] Finished product analysis
[0076] The composition of extract and raffinate was analyzed by chiral column Chiralcel-OJ. The content of L-donepezil or D-donepezil is 100%, 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com