Asymmetric fluoreno imidazole derivative and preparation method thereof
A technology of fluorenimidazole and derivatives, which is applied in the field of asymmetric fluorenimidazole derivatives and their preparation, to achieve the effects of enhancing blue light emission performance, overcoming poor chemical stability, and overcoming solubility reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
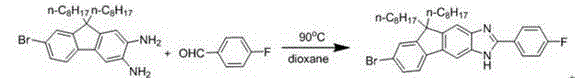
[0034]
[0035] Dissolve 1g of 2,3-diamino-7-halo-9,9′-dioctylfluorene and 1 times the molar amount of benzaldehyde in 20mL of dioxane, react at 90°C for about 10 hours, stop After the reaction, cooling to remove the solvent, separation and purification by column chromatography gave 0.94 g of light yellow solid (yield: 80%). 1HNMR (500M, CDCl 3 ): δ(ppm) 8.18-8.09(m, 2H),7.68(s, 1H),7.58-7.37(m, 7H),2.03-1.90(m, 4H),1.24-0.98(m, 20H),0.86 -0.56(m, 10H).
Embodiment 2
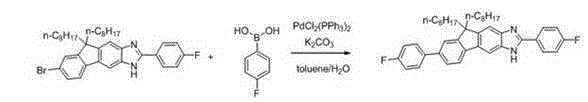
[0037]
[0038] Add 100 mg of bromofluorenimidazole compound, 1.2 times the molar amount of phenylboronic acid, 2 times the molar amount of potassium carbonate and 0.05 molar amount of bistriphenylphosphine palladium dichloride to toluene (8 mL) and water (1 mL) In a mixed solvent, under the protection of nitrogen, the reaction was carried out at 90°C for 12 hours, the reaction was stopped and cooled to room temperature, and the solvent was removed, followed by column chromatography separation and purification to obtain 90 mg of light yellow solid (yield: 90%). 1 HNMR (500M, CDCl 3 ): δ(ppm)8.17(d, J =8.0 Hz,2H),7.85(s, 1H),7.75-7.67(m, 3H),7.66-7.54(m, 3H),7.53-7.44(m, 5H),7.40-7.35(m, 1H), 2.07-1.97(m, 4H), 1.22-0.98(m, 20H), 0.84-0.67(m, 10H). HR-MS (ESI, m / z): [M+H] + calcd for (C 42 h 50 N 2 ) 583.4052; found, 583.4038.
[0039] The excitation spectrum and emission spectrum of the compound were measured by a fluorescence spectrophotometer, and the optimum excit...
Embodiment 3
[0041]
[0042] Add 100 mg of bromofluorenimidazole compound, 1.5 times the molar amount of naphthaleneboronic acid, 2 times the molar amount of potassium carbonate and 0.05 molar amount of bistriphenylphosphine palladium dichloride to toluene (12 mL) and water (1 mL) In a mixed solvent, under the protection of nitrogen, react at 90°C for 12 hours, stop the reaction and cool to room temperature. After removing the solvent, column chromatography separates and purifies 98 mg of yellow solid (yield: 91%). 1 HNMR (500M, CDCl 3 ): δ(ppm) 8.26(d, J =7.5 Hz, 2H),8.05-7.82(m, 4H),7.76(d, J =8.0 Hz, 1H), 7.63-7.41(m, 10H), 2.13-1.92(m, 4H), 1.22-1.03(m, 20H), 0.91-0.69(m, 10H). HR-MS (ESI, m / z): [M+H] + calcd for (C 46 h 52 N 2 ) 632.4208; found, 632.4220.
[0043] The excitation spectrum and emission spectrum of the compound were measured by a fluorescence spectrophotometer, and the optimum excitation wavelength was determined to be 359nm, and the maximum emission wavelengt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com