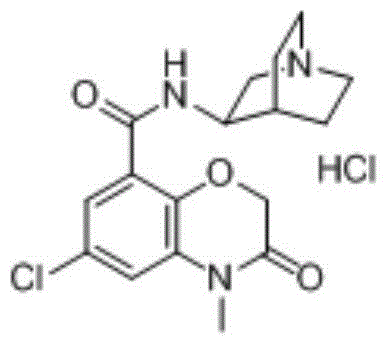
Method for preparing azasetron hydrochloride
A technology of azasetron hydrochloride and aminoquinuclidine hydrochloride, which is applied in the field of medicine, can solve problems such as difficult removal of by-products, environmental and equipment pollution, unfavorable production methods, etc., to achieve mild reaction conditions, avoid pollution, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
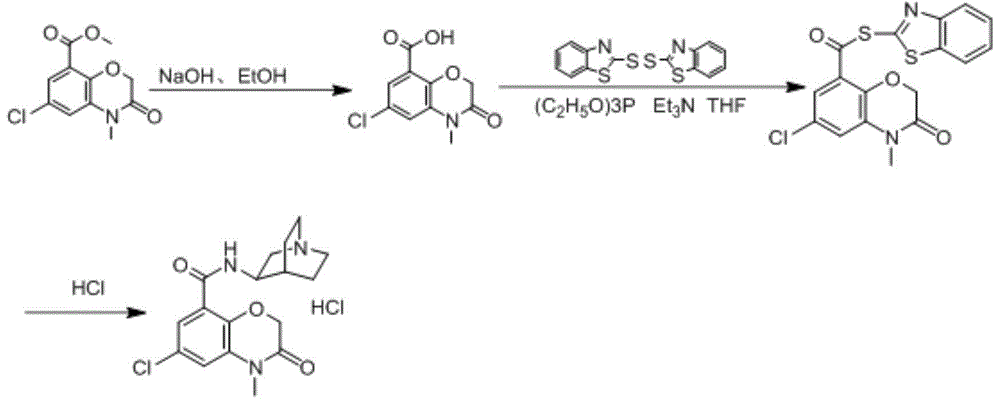
[0080] In a 500ml three-necked flask, add 150ml of water and 12g (0.3mol) of sodium hydroxide, stir until the solid is completely dissolved, then add 100ml of ethanol, cool to 20℃, add 6-chloro-4-methyl-3,4-bis Hydrogen-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester 25.5g (0.1mol), stirred and reacted for 3h, the reaction was over, cooled below 10℃, diluted hydrochloric acid to adjust the pH to 2 , Filtered and dried to obtain 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid as an off-white solid, 21.9g, yield The rate is 91%.
[0081] 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid (12.0g, 0.05mol), triethyl phosphite ( 10.0g, 0.062mol), 2,2'-dithiodibenzothiazole (20.0g, 0.062mol) and tetrahydrofuran (100ml) were added to the dry reaction flask, stirred at room temperature for 1h, and the temperature was controlled below 20℃ in an ice-water bath. A solution of triethylamine (8.6ml) in tetrahydrofuran (20ml) was added d...
Embodiment 2
[0087] In a 500ml three-necked flask, add 200ml of water and 12g (0.3mol) of sodium hydroxide, stir until the solid is completely dissolved, then add 100ml of ethanol, cool to 21℃, add 6-chloro-4-methyl-3,4-bis Hydrogen-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester 25.5g (0.1mol), stirred for 3h, the reaction is over, cooled below 10℃, dilute hydrochloric acid adjusts the pH to 1 ~2, filter and dry to obtain 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid as a white solid, 20.9g , The yield is 86.7%.
[0088] 6-Chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid (12.0g, 0.05mol), triethyl phosphite ( 9.7g, 0.06mol), 2,2'-dithiodibenzothiazole (19.4g, 0.06mol) and tetrahydrofuran (100ml) were added to the dry reaction flask, stirred at room temperature for 1h, and the temperature was controlled below 20℃ in an ice-water bath. A solution of triethylamine (8.6ml) in tetrahydrofuran (20ml) was added dropwise. After dripping, re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com