Armadillidiam vulgare plasmin and application thereof
A technology of fibrinolytic enzymes and fibrinolytic enzymes, applied in fibrinolytic enzymes and its application fields, can solve the problems that cannot be used to prevent thrombosis and poor anticoagulant activity, and achieve the reduction of the risk of tissue hemorrhage, strong thrombolysis and anticoagulation Mild effect of coagulation and thrombolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of murine fibrinolytic enzyme
[0041] Murine fibrinolytic enzyme of the present invention adopts following method to prepare:
[0042] (1) Wet ultrafine pulverization extraction
[0043] Weigh a certain amount of dried muffins, add 8 times the mass of muffins, soak in distilled water at 25°C for 1 h, put them into an ultrafine pulverizer and pulverize them at 25°C for 10 min. The obtained slurry was heated at 5000 rpm at 4 °C -1 Centrifuge for 10 min and take the supernatant to obtain the extract.
[0044](2) Use hollow fiber membrane to extract protein components with a molecular weight of 4kDa~80kDa
[0045] Pass the extract obtained in step (1) through a PVDF (polyvinylidene fluoride) hollow fiber membrane with a molecular weight cut-off of 80kDa, the operating pressure is 21.34 kPa ~ 21.56kPa, the flow rate is 8ml / min, and the permeate is taken. The permeate is passed through a PES (polyethersulfone) hollow fiber membrane with a molecu...
Embodiment 2
[0053] Example 2. Purification results of murine plasmin PSLTro01
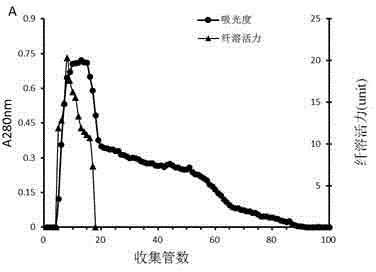
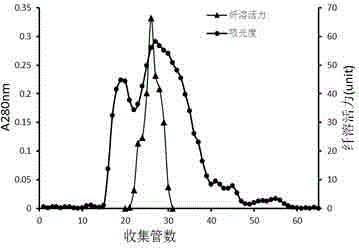
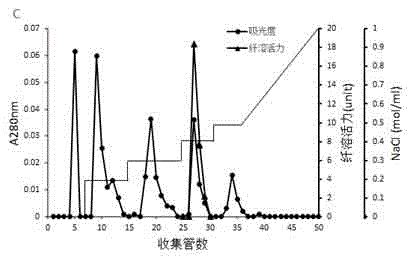
[0054] Adopt the result of method purification in embodiment 1 as Figure 1-4 shown. The separation range of the Sephadex G-100 gel filtration column is 2 kDa-120 kDa, and the chromatogram is as follows figure 1 As shown, a large elution peak and a longer shoulder peak appeared during the elution process, and the protein with thrombolytic activity flowed out of the column before other components, suggesting that the molecular weight of the protein with thrombolytic activity may be greater than 10 kDa. figure 2 The chromatogram of the Sephacryl S-200 HR gel filtration column is shown, and there are two main parallel peaks, and the protein with thrombolytic activity is mainly concentrated in the front part of the second eluting peak. During the elution process of HiTrap Capto Q ion-exchange chromatography column, there are 5 different protein elution peaks, breakthrough peak, low salt elution (the NaCl conce...
Embodiment 3
[0063] Example 3 Determination of the purity, relative molecular mass and N-terminal amino acid sequence of plasminase PSLTro01
[0064] 1. Purity test
[0065] (1) Purity detection by non-reducing SDS-PAGE vertical electrophoresis
[0066] The concentration of the separating gel in the non-reducing SDS-PAGE vertical electrophoresis was 12%, and the concentration of the stacking gel was 3.9%. Take 10 µL of the plasminase PSLTro01 prepared in Example 1 and 2 µL of non-reducing loading buffer (purchased from: Beyond Biotechnology Research Institute), mix thoroughly, and boil at 100°C for 5 min. Electrophoresis was started at a constant voltage of 80 V. After the front of the bromophenol blue band entered the separation gel, the voltage was adjusted to 120 V until the bromophenol blue band reached the bottom of the separation gel. After electrophoresis, silver staining was performed quickly. Such as Figure 5 As shown, only a single electrophoresis band was displayed in the no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com