A low-molecular-weight heparin sustained-release preparation and its preparation method and application
A low-molecular-weight heparin and sustained-release preparation technology, applied in the field of medicine, can solve the problems affecting the in vivo stability and therapeutic efficiency of the preparation, the rapid and mass release of low-molecular-weight heparin, poor biocompatibility, etc., and achieve prolonging blood half-life and in vivo stability. Excellent performance and improved compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In this embodiment, low molecular weight heparin sustained-release preparations are prepared by the following method, which includes the following steps:
[0053] Weigh 100mg polylysine (PLL, M W =13000Da) into a centrifuge tube, add 5mL of 5% glucose solution, and mix by ultrasonic; Measure 5mL of 15mg / mL PLL solution in a centrifuge tube, then add 5mL of 6mg / mL Traut's reagent, add a magnet, and place under a magnetic force Stir on the stirrer for 2h to carry out mercaptolation to prepare a mercaptolated PLL (PLL-SH) solution; add 60 mL of 5% glucose solution to the mercaptolated PLL (PLL-SH) solution and mix evenly, and quickly add to the mixed solution Add 100mL of 20mg / mL sodium heparin (LMWH) solution, mix well, homogenize, age for 24h to stabilize the nanoparticles, freeze-dry, and the reconstituted preparation solution is used for subsequent characterization.
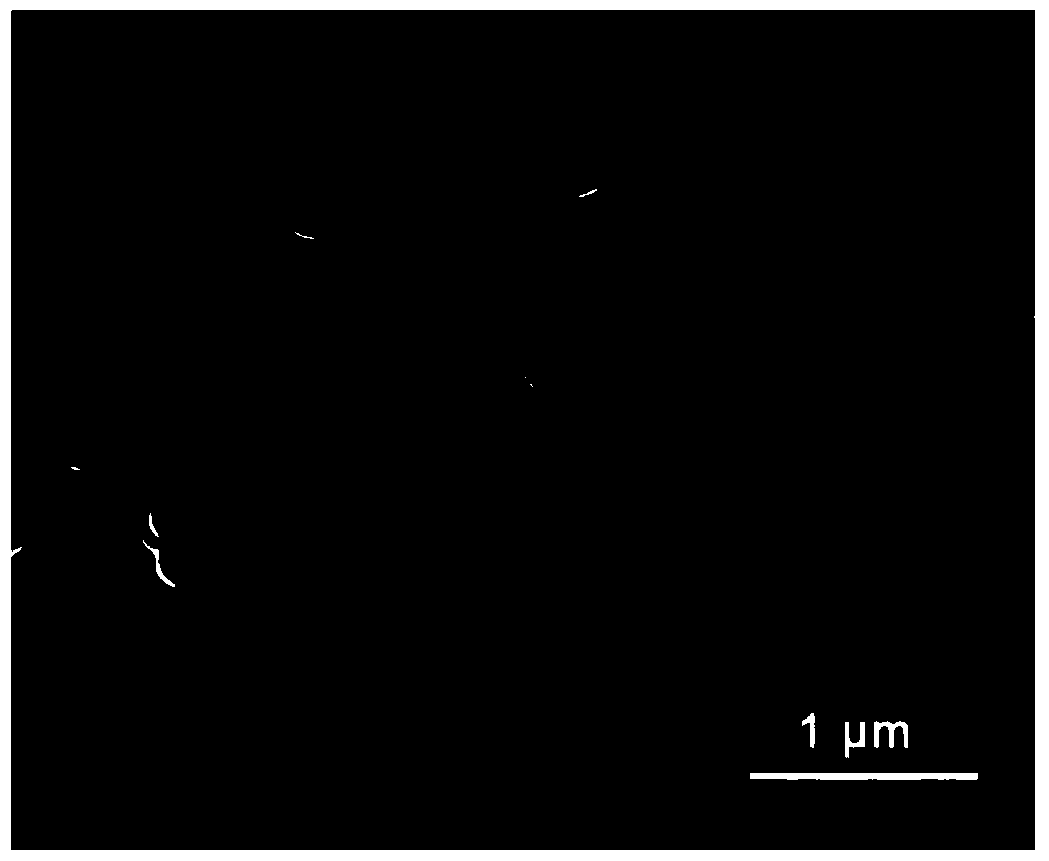
[0054] The nanoparticles prepared in this embodiment were characterized by scanning electron microsco...
Embodiment 2
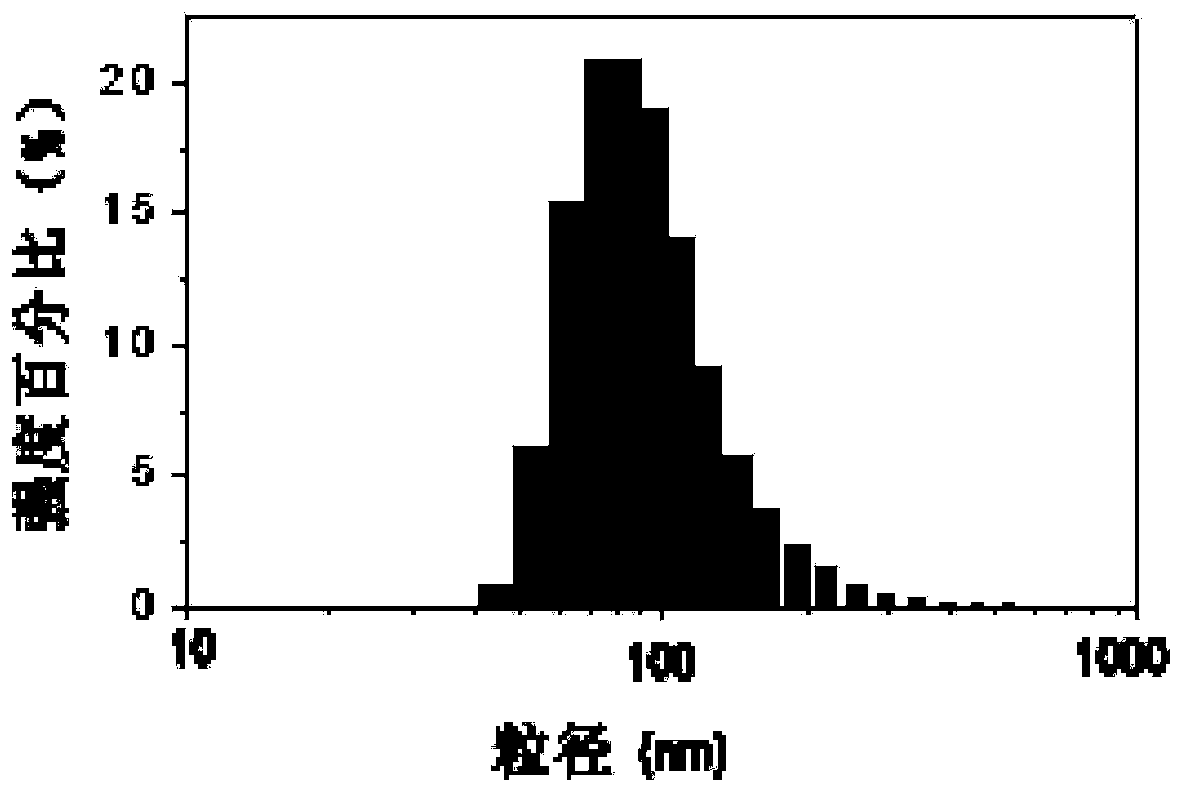
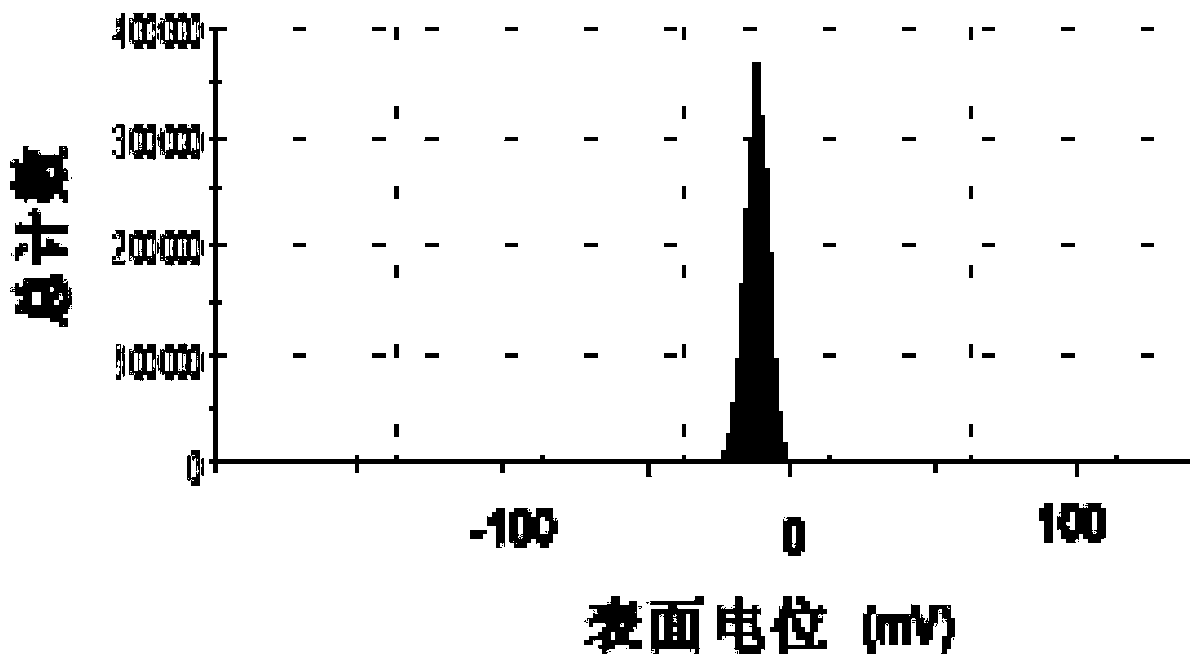
[0056] The difference between this example and Example 1 is that the mass ratio of PLL to low molecular weight heparin is adjusted to 5:6, and the rest of the preparation methods and conditions are the same as in Example 1. The prepared low molecular weight heparin sustained-release preparation nanoparticles The particle size and potential of nanoparticles were determined by dynamic light scattering technique (DLS).
[0057] The result is shown in Figure 2, where Figure 2A is the particle size distribution diagram of the low molecular weight heparin sustained-release preparation, as can be seen from the figure, the average particle size is about 105nm; Figure 2B It is the surface potential map of low molecular weight heparin sustained-release preparation; as shown in the figure, the surface potential is about -16mV.
Embodiment 3
[0059] In this embodiment, low molecular weight heparin sustained-release preparations are prepared by the following method, which includes the following steps:
[0060] Weigh 100mg polyhistidine (M W =1000Da) into a centrifuge tube, add 5mL of 5% glucose solution, and mix by ultrasonic; Measure 5mL of 20mg / mL PLL solution in a centrifuge tube, then add 0.1mg / mL Traut's reagent 10mL, add magnets, place Stir on a magnetic stirrer for 2 hours to carry out mercaptolation to obtain a mercaptolylated polyhistidine solution; add 50 mL of 5% glucose solution to the mercaptolylated polyhistidine solution and mix well, and quickly add 5 mL of 30 mg / mL dalteparin sodium (LMWH) solution, homogenized after mixing, aged for 24 h to stabilize the nanoparticles, and freeze-dried to prepare low molecular weight heparin sustained-release preparations.
[0061] Characterized by scanning electron microscopy and dynamic light scattering, the low molecular weight heparin sustained-release prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com