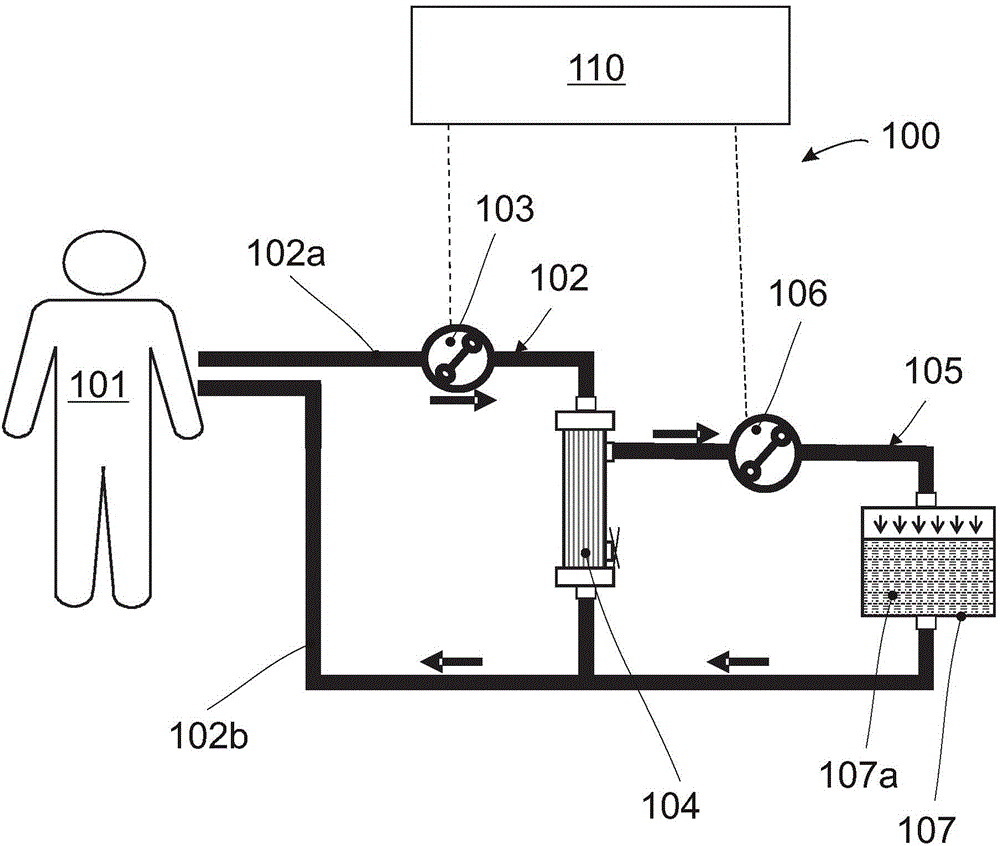
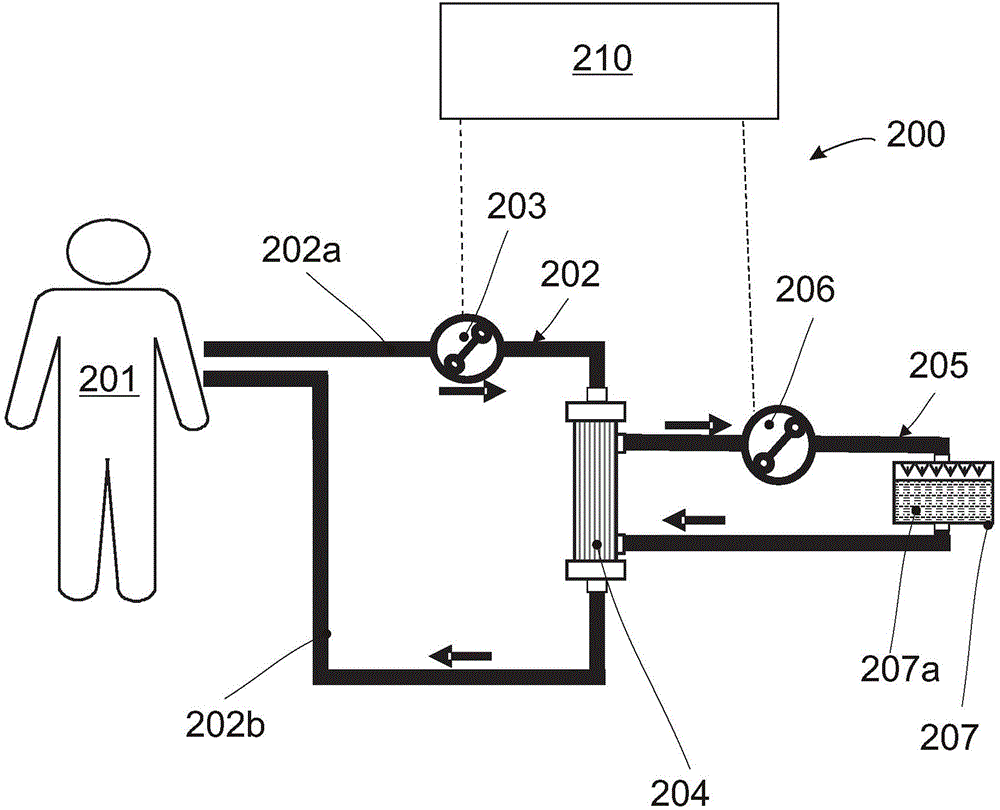
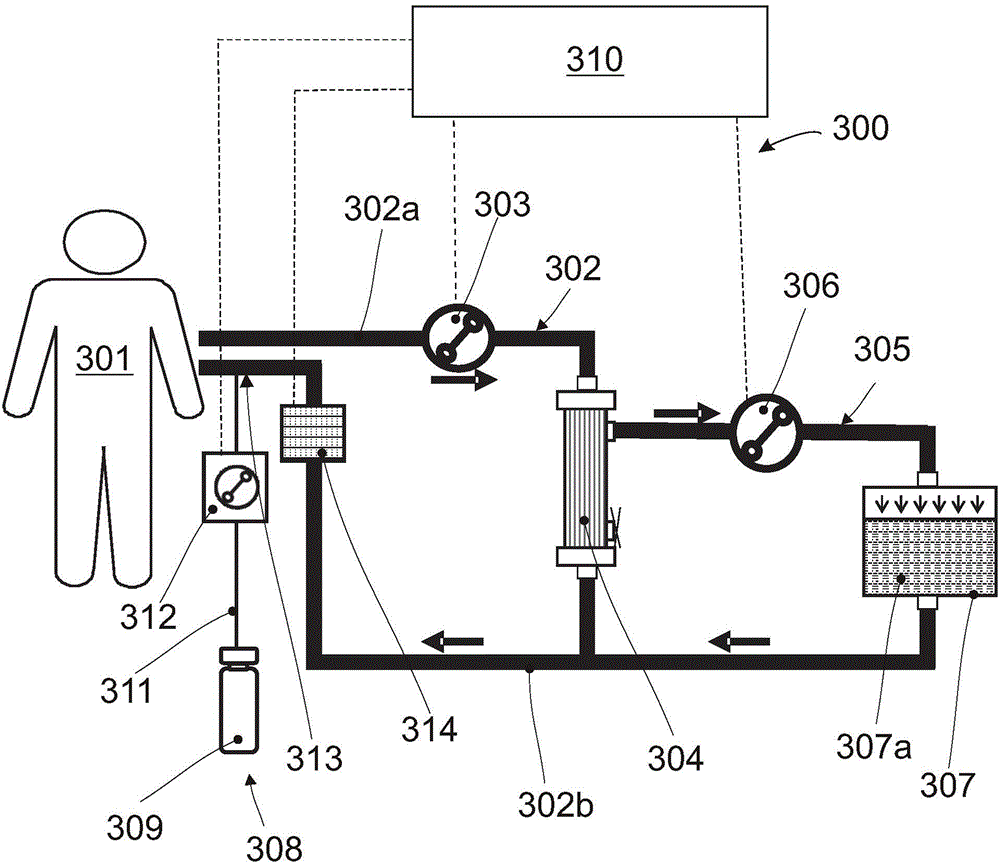
Extracorporeal perfusion apparatus
A technology of in vitro perfusion and equipment, which can be used in extracellular fluid diseases, alkali metal oxides/hydroxides, inorganic chemistry, etc., and can solve the problem of high mortality of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0127] 1. Example 1: Differently with polymyxin B (PMB) coated carrier (average particle size: 120 microns, average pore size: 15-20 nm) PMB in plasma and separated plasma (using Albuflow filter ) desorption in
[0128] 1.1PMB coating
[0129] Support: Amberchrom CG161c (polystyrene-divinylbenzene copolymer, The Dow Chemical Company), with an average particle size of 120 micrometers and an average pore size of 15 nanometers; accessible surface is 900 square meters per gram of polymer (dry) rice. The dry weight per milliliter of wet carrier was 18% (weight / volume).
[0130] Polymyxin B (PMB): Polymyxin B sulfate (Sigma Aldrich)
[0131] The PMB solution (10 mg / ml in distilled water) was autoclaved at 121° C. for 30 minutes, and the carrier was then coated with PMB in a 15 ml Greiner tube as follows (Table 1.1): 3 ml carrier with 7.5 ml PMB solution
[0132] Table 1.1
[0133]
[0134] The coating was performed overnight on a tumble mixer at room temperature. The car...
example 2
[0145] 2. Example 2: Endotoxin batches in serum with different PMB-coated carriers
[0146] 2.1 Test structure
[0147] The conditioned carrier (Amberchrom CG161c: Ethylvinylbenzene-divinylbenzene copolymer (The Dow Chemical Company), with an average particle size of 120 μm and an average pore size of 15 nm) was treated with different amounts of polymyxin B (PMB) as coating: 0, 5, 10, 15 and 25 mg per gram of wet carrier. These were tested for inactivation of LPS in serum in an endotoxin batch test in triplicate.
[0148] 2.2 Execution of the test
[0149] PMB coating:
[0150] Samples of the vehicle were produced at different PMB concentrations (5 mg, 10 mg, 15 mg and 25 mg per gram of wet vehicle) (see protocol in Example 1 above). PMB solution (10 mg / ml in distilled water) with the carrier in a 50% suspension was autoclaved at 121°C for 30 minutes, and the carrier was coated with PMB in a 15 ml Glenner tube as follows (Table 2.2) :
[0151] Table 2.2:
[0152] ...
example 3
[0161] 3. Example 3: Inactivation of endotoxin (LPS) according to the concentration of polymyxin based on endotoxins from Escherichia coli and Pseudomonas aeruginosa.
[0162] 3.1. Goals
[0163] The objective of this test is to determine the inactivation of endotoxin in plasma as a function of polymyxin B (PMB) concentration (batch test I). Furthermore, it was investigated to what extent this endotoxin inactivation leads to inhibition of cytokine diffusion (batch test II).
[0164] 3.2. Blood donors
[0165] Nine blood sample tubes (9 ml each) spiked with 5 IU of heparin were taken from the donor. Plasma was separated by centrifuge and cell pellets were incubated on a roller mixer. Endotoxin (LPS) spiked into plasma used for batch test I:
[0166] 3.3. Incorporation of LPS, polymyxin B solution and batch test I
[0167] LPS: Pseudomonas aeruginosa (L-7018Sigma Lot: 128K4115, -70°C, 100 μl 10 -3 g / mL (1 mg / mL))
[0168] LPS: Escherichia coli (L-4130Sigma Lot: 110M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com