Catalyst for ultraviolet photocatalytic degradation of organic pollutants and preparation method thereof
A technology for the catalytic degradation of organic pollutants, applied in the field of preparation of catalysts for the degradation of organic pollutants by ultraviolet light, can solve the problems of harsh reaction conditions, low catalytic efficiency, long reaction time, etc. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preparation method of a catalyst for photodegradation of organic pollutants, comprising the following steps:
[0036] A. Pour 8-15g tetrabutyl titanate (TBT), 5-10ml acetic acid, 100-140ml methanol and 5-7g polyvinylpyrrolidone (PVP) into a 250mL conical flask, stir at room temperature for 4-8h, Obtain PVP / TBT sol;
[0037] B. Electrospin the PVP / TBT sol to obtain a PVP / TBT nanofiber membrane;
[0038] C. The obtained PVP / TBT nanofiber membrane was calcined at 500 °C for 4 h to obtain TiO 2 Nanofibers;
[0039] D. 50mg TiO 2 Disperse evenly into a quartz bottle containing 5-50mL of 0.001mol / L silver nitrate solution, and stir for 2h in the dark to make the silver ions in the solution adsorb to TiO 2 nanofiber surface;
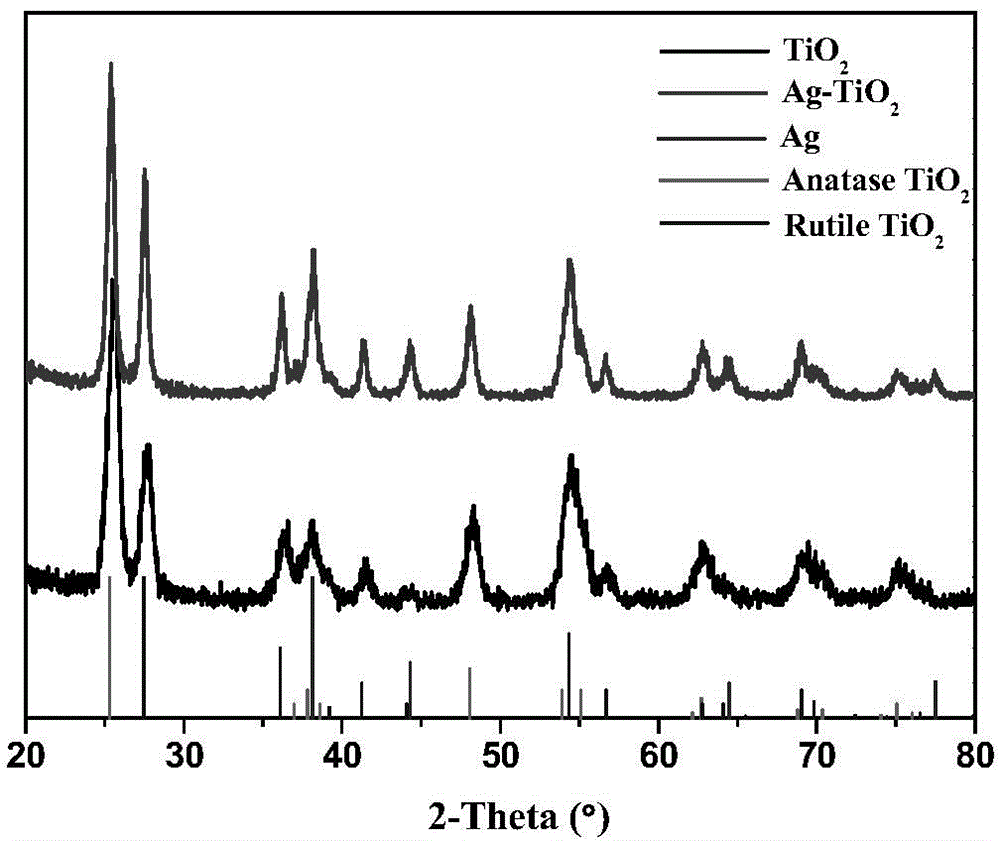
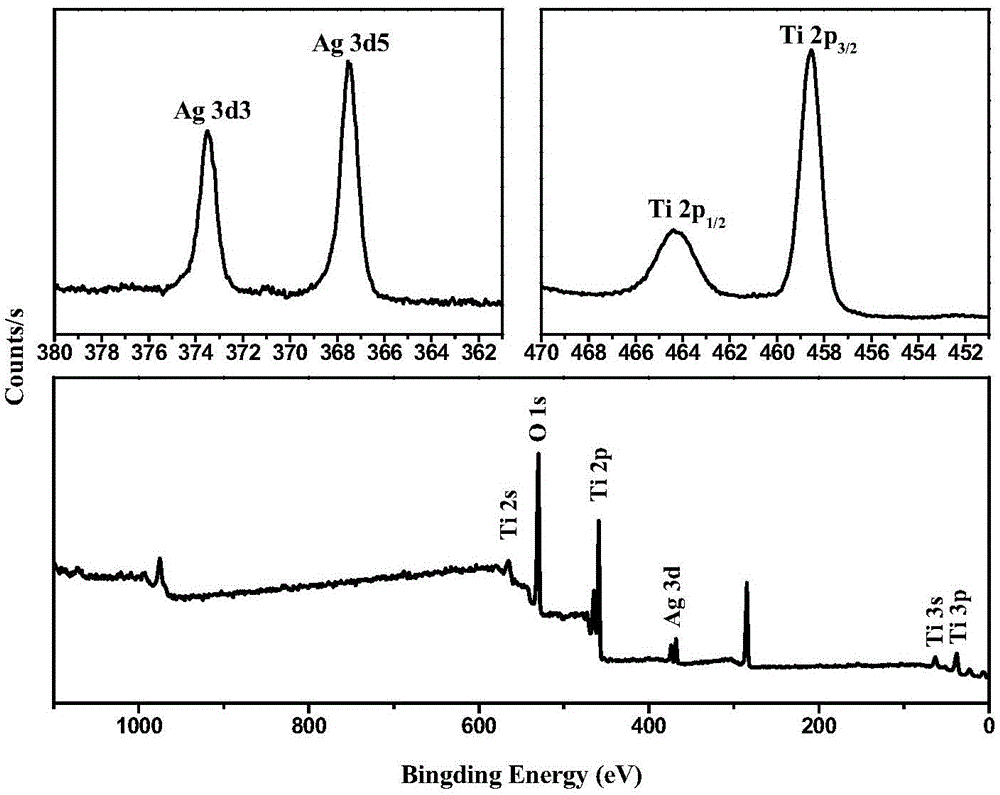
[0040] E. TiO adsorbed with silver ions 2 The nanofibers were irradiated with ultraviolet light for 30min-1h, filtered, washed and dried to obtain Ag / TiO 2 catalyst.
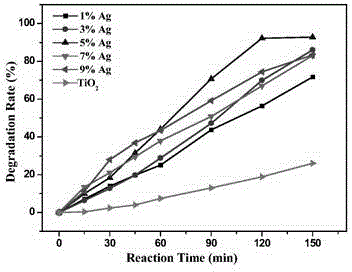
[0041] The silver loading is 1-9%.
[0042] described in TiO 2 Loading the...
Embodiment 1
[0044] A. Pour 10g of tetrabutyl titanate (TBT), 7mL of acetic acid, 120mL of methanol and 5.6g of polyvinylpyrrolidone (PVP) into a 250mL conical flask, and stir at room temperature for 4h to obtain a PVP / TBT sol;
[0045] B, will electrospin the PVP / TBT sol to obtain the PVP / TBT nanofiber membrane;
[0046] C. The obtained PVP / TBT nanofiber membrane was calcined at 500 °C for 4 h to obtain TiO 2 Nanofibers;
[0047] D. 50mg TiO 2 Disperse evenly into a quartz bottle containing 5.25mL of 0.001mol / L silver nitrate solution, and stir in the dark for 2 hours to make the silver ions in the solution adsorb to TiO 2 nanofiber surface;
[0048] E. TiO adsorbed with silver ions 2 The nanofibers were irradiated with ultraviolet light for 45 min, filtered, washed, and dried in vacuum at room temperature to obtain 1% Ag / TiO 2 catalyst.
Embodiment 2
[0050] A. Pour 8g tetrabutyl titanate (TBT), 5ml acetic acid, 100ml methanol and 5g polyvinylpyrrolidone (PVP) into a 250mL conical flask, and stir at room temperature for 7h to obtain PVP / TBT sol;
[0051] B, will electrospin the PVP / TBT sol to obtain the PVP / TBT nanofiber membrane;
[0052]C. The obtained PVP / TBT nanofiber membrane was calcined at 500 °C for 4 h to obtain TiO 2 Nanofibers;
[0053] D. 50mg TiO 2 Disperse evenly into a quartz bottle containing 15.75mL of 0.001mol / L silver nitrate solution, and stir in the dark for 2 hours to make the silver ions in the solution adsorb to TiO 2 nanofiber surface;
[0054] E. TiO adsorbed with silver ions 2 The nanofibers were irradiated with ultraviolet light for 30 min, filtered, washed, and dried to obtain 3% Ag / TiO 2 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com