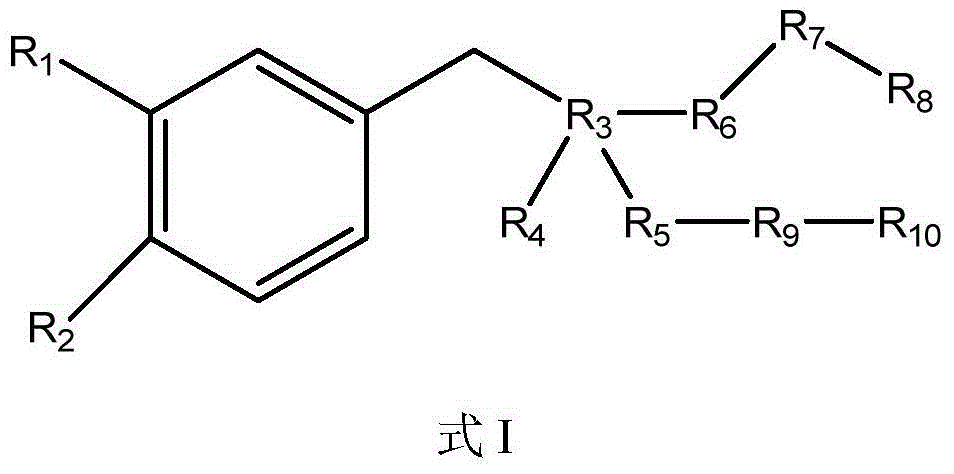
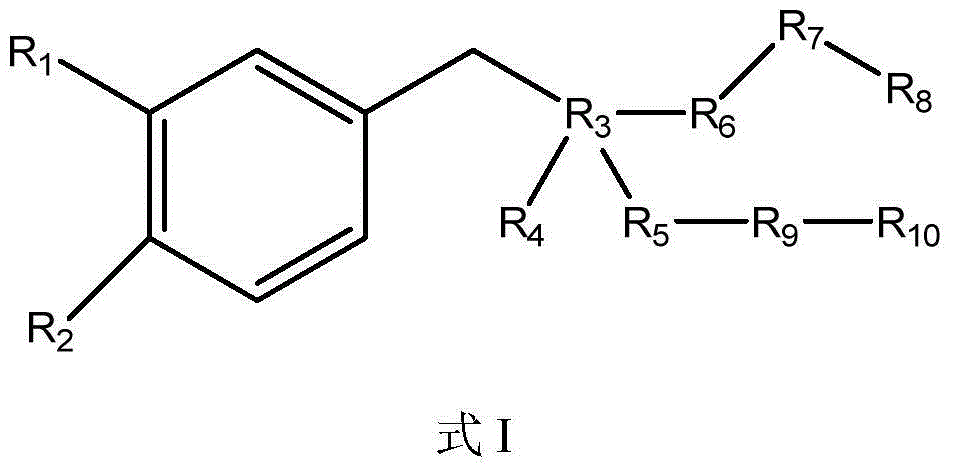
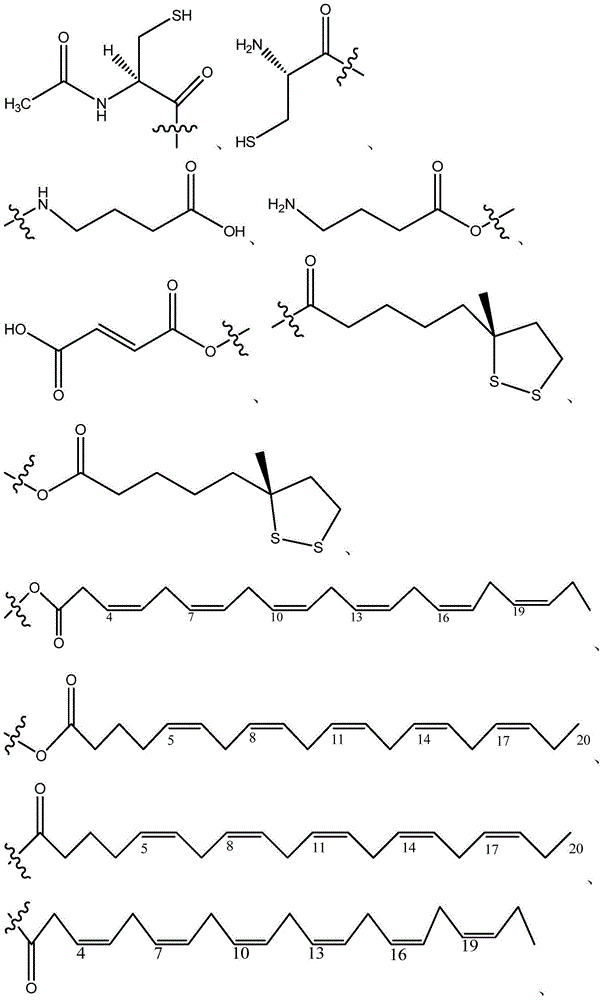
Compositions and methods for treatment of neuromuscular disorders and neurodegenerative disorders
A composition and compound technology, applied in neuromuscular system diseases, muscular system diseases, nervous system diseases, etc., can solve problems such as pessimism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161]
[0162] Synthesis of Compound-2:
[0163]
[0164] To compound 1 (50 mmol) in 1:1 THF / H at 0 °C 2 To a solution in O mixture (300 mL), NaHCO was added continuously 3 (150mmol) and Boc 2 O (110 mmol). After 30 minutes, the solution was stirred at room temperature overnight. with Et 2 O extracted the resulting cloudy solution (2 x 200 mL). Half-saturated citric acid was added carefully at 0°C, the aqueous layer was acidified to pH=4-5, then washed with CH 2 Cl 2 Extraction (3 x 200 mL). The combined organic phases were dried (Na 2 SO 4 ) and evaporated under reduced pressure to give high-purity Boc-amino acid in the form of a solid or hard colorless oil, which was allowed to crystallize on standing. These Boc-amino acids were used in the next step without purification. M.F: C 20 H 30 N 2 O 8 ; Mol. Wt: 426.46.
[0165] Synthesis of Compound-3:
[0166]
[0167] Compound 2 (50 mmol), 2,2-dimethoxypropane (200 mmol) and anhydrous toluene (20 vol) ...
Embodiment 2
[0175]
[0176] Synthesis of Compound-2:
[0177]
[0178] Compound 1 (50 mmol), 2,2-dimethoxypropane (200 mmol) and anhydrous toluene (20 vol) were added to a 500 mL two-necked flask. One neck of the flask is equipped with a Soxhlet extractor, the cannula of which is filled with granular anhydrous CaCl 2 (75g) to capture MeOH and H 2 O. The other neck of the flask was sealed with a sampling septum. After the system was flushed with argon for 5 minutes and heated to reflux for 5 minutes, p-toluenesulfonic acid monohydrate (430 mg, 4.5 mol%) was added and left to reflux for 12 hours. The reaction mixture was concentrated under reduced pressure. The reaction residue was diluted with ethyl acetate (10 Vol) and washed with saturated sodium bicarbonate solution followed by brine. The organic layer was dried over sodium sulfate, filtered and concentrated in vacuo to yield compound 2. M.F: C 16 H 22 N 2 O 4 ; Mol. Wt: 306.36.
[0179] Synthesis of Compound-4:
[0180]...
Embodiment 3
[0188]
[0189] Synthesis of Compound-2:
[0190]
[0191] To a solution of compound 1 (50 mmol) in dichloromethane (10 mL) at 0°C were successively added diisopropylethylamine (150 mmol) and methoxyethoxymethyl chloride (MEM-Cl) (110 mmol). The reaction mixture was stirred for 3.0 hours, monitored by TLC for completion, and washed with water (10 Vol) and brine. The organic phase was dried (Na 2 SO 4 ) and evaporated under reduced pressure to form the benzaldehyde derivative 2. M.F: C 19 H 30 O 10 ; Mol. Wt: 418.44.
[0192] Synthesis of Compound-3:
[0193]
[0194] A Soxhlet extractor was filled with 20 g molecular sieves and the benzaldehyde derivative (5 mmol) and hydrazine hydrate (75 mmol) were refluxed in ethanol (10 vol) overnight (16-24 hours). The hot alcoholic solution was filtered and the solvent was evaporated to yield (73%) crude product-3. Compound 3 was formed after one recrystallization from ethanol. M.F: C 19 H 32 N 2 O 9 ; Mol. Wt: 43...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com