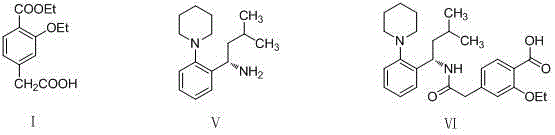
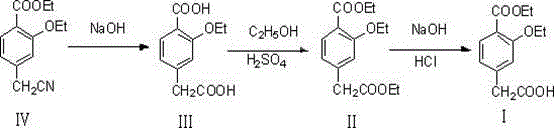
Method for synthesizing repaglinide key intermediate
An intermediate and key technology, which is applied in the field of synthesizing key intermediates of repaglinide and preparing hypoglycemic drugs, can solve the problems of unsuitability for large-scale industrial production, complex process, long production cycle, etc., and achieve complete reaction and yield The effect of high rate and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for synthesizing repaglinide key intermediate 4-ethoxycarbonyl-3-ethoxyphenylacetic acid, the process steps are as follows:
[0026] 1. Synthesis of compound (Ⅲ) 4-carboxymethyl-2-ethoxy-benzoic acid
[0027] Add 270g of drinking water to the reaction bottle, start stirring, put 50g of compound (Ⅳ), heat up to 50°C, start to drop 120g of 20% liquid caustic soda, after dropping, raise the temperature to 75~80°C, and keep warm for 3.5 hours. After the heat preservation is completed, lower the temperature to 50-60°C, slowly add about 48g of hydrochloric acid dropwise, and adjust the pH to 5-6. Add 5g of medicinal charcoal, decolorize for 30 minutes, filter with suction, and add about 47g of hydrochloric acid dropwise to the filtrate at 50~60°C to adjust pH=3.0~3.5. Cool down to 5-10°C, filter and dry to obtain 46.4 g of compound (Ⅲ), with a yield of 96.5% and a purity of 98.48%.
[0028] 2. Synthesis of compound (II) 3-ethoxy-4-ethoxycarbonyl-ethyl phenylacetate...
Embodiment 2
[0033] A method for synthesizing repaglinide key intermediate 4-ethoxycarbonyl-3-ethoxyphenylacetic acid, the process steps are as follows:
[0034] 1. Synthesis of compound (Ⅲ) 4-carboxymethyl-2-ethoxy-benzoic acid
[0035] Add 270g of drinking water to the reaction bottle, start stirring, put 50g of compound (Ⅳ), heat up to 50°C, start to drop 240g of 10% liquid caustic soda, after dropping, raise the temperature to 80~85°C, and keep warm for 5.0 hours. After the heat preservation is completed, lower the temperature to 50-60°C, slowly add about 48g of hydrochloric acid dropwise, and adjust the pH to 5-6. Add 5g of medicinal charcoal, decolorize for 30 minutes, filter with suction, add about 47g of hydrochloric acid dropwise to the filtrate at 50~60°C, and adjust the pH to 1.0~2.0. Cool down to 5-10°C, filter and dry to obtain 45.5 g of compound (Ⅲ), with a yield of 94.7% and a purity of 98.15%. The reaction time is long and the purity is low.
[0036] 2. Synthesis of compou...
Embodiment 3
[0041] A method for synthesizing repaglinide key intermediate 4-ethoxycarbonyl-3-ethoxyphenylacetic acid, the process steps are as follows:
[0042] 1. Synthesis of compound (Ⅲ) 4-carboxymethyl-2-ethoxy-benzoic acid
[0043] Add 270g of drinking water into the reaction bottle, start stirring, put 50g of compound (Ⅳ), heat up to 50°C, start to drop 120g of 20% liquid caustic soda, after dropping, raise the temperature to 80~85°C, and keep warm for 2.5~3.0 hours. After the heat preservation is completed, lower the temperature to 50-60°C, slowly add about 48g of hydrochloric acid dropwise, and adjust the pH to 5-6. Add 5g of medicinal charcoal, decolorize for 30 minutes, filter with suction, add about 47g of hydrochloric acid dropwise to the filtrate at 50~60°C, and adjust the pH to 2.0~2.5. Cool down to 5-10°C, filter and dry to obtain 47g of compound (Ⅲ), with a yield of 97.8% and a purity of 98.76%. The yield and quality both reached a high level.
[0044] 2. Synthesis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com