Method for controlling racemization rate of dihydromyricetin
A technology of dihydromyricetin and racemization rate is applied in the field of controlling the racemization rate of dihydromyricetin in the extraction process, and can solve problems such as blindness and poor stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
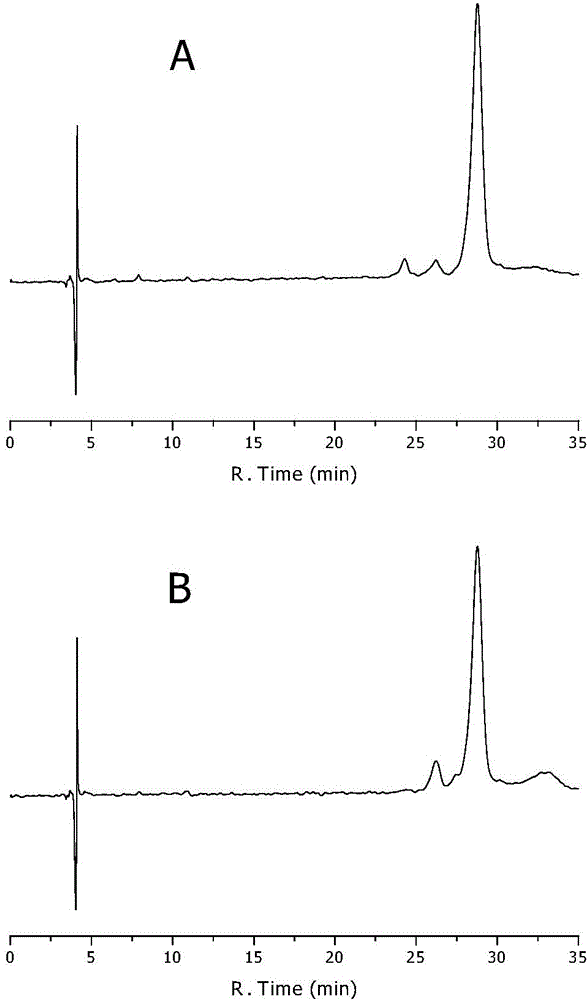
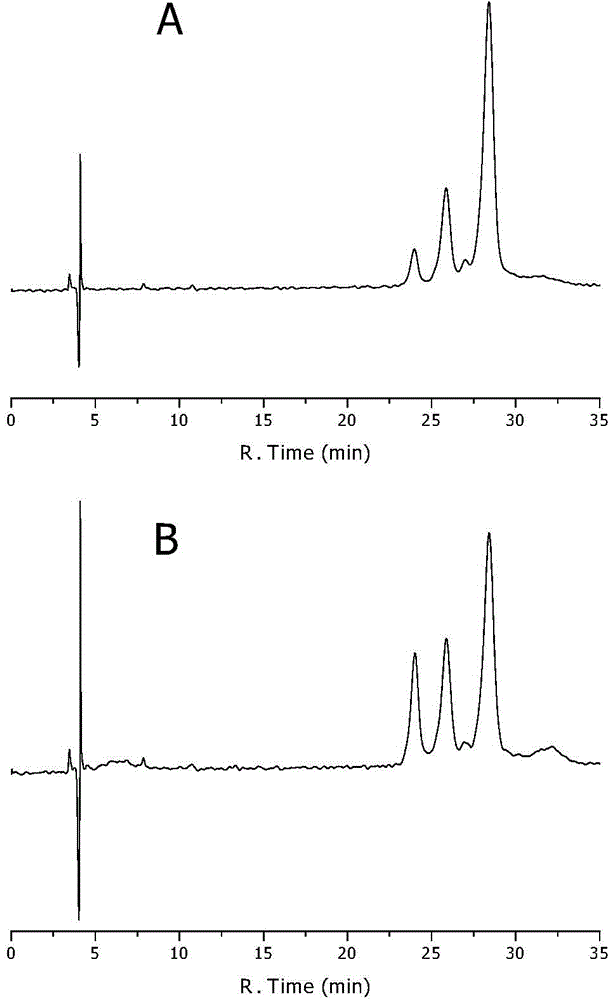
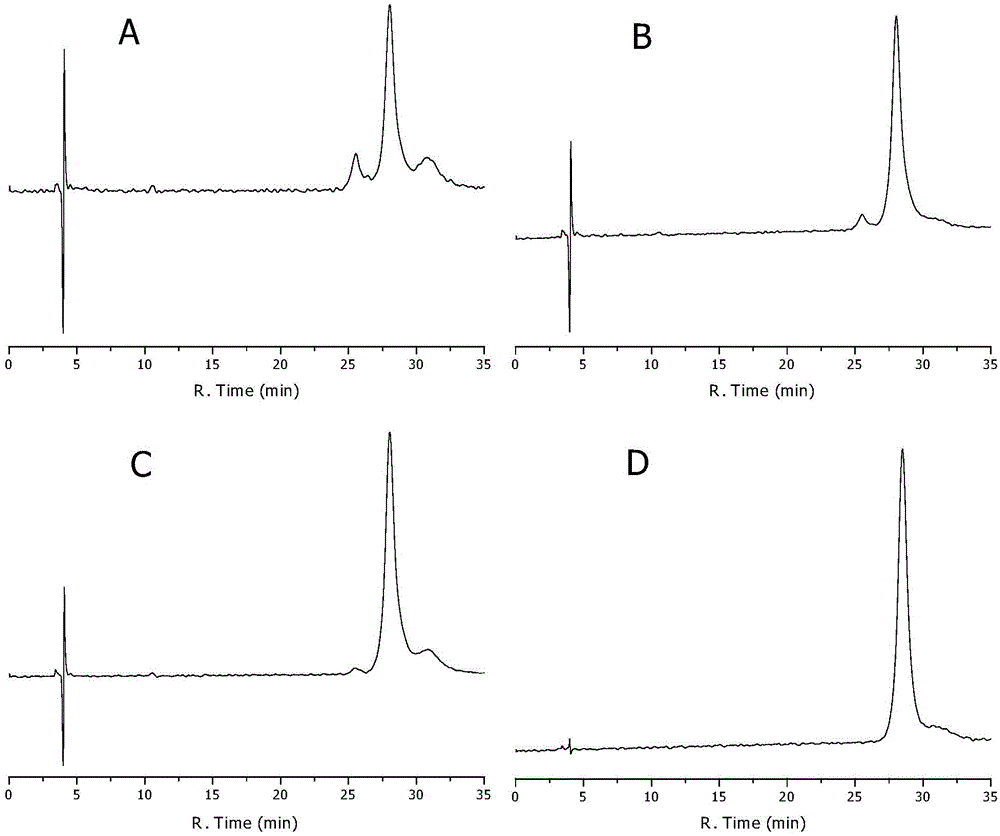
[0082] Example 1 Construction and Verification of the Optical Purity Method for Measuring Dihydromyricetin by RP-HPLC
[0083] The present invention studies and establishes a chiral mobile phase additive RP-HPLC separation method for dihydromyricetin enantiomers, and detects the optical purity of dihydromyricetin during the extraction process. In the method, a chiral additive is added into a chromatographic mobile phase system composed of an organic phase and an aqueous phase, and the enantiomers of dihydromyricetin can be effectively separated.
[0084] Instruments and conditions: Waters e2695 high performance liquid chromatography (with Waters 2998 photodiode array detector), octadecylsilane bonded silica gel as filler; methanol: β-cyclodextrin solution (take β-cyclodextrin 18g, phosphoric acid 10ml, add water 1000ml to dissolve) 10:90 as the mobile phase; the flow rate is 0.8ml per minute; the column temperature is 30°C; the ultraviolet detection wavelength is 290nm (the sc...
Embodiment 2
[0088] Example 2 Extracting dihydromyricetin from vine tea
[0089] Take the stems and leaves of vine tea, add 5 times the amount of 75% ethanol (hydrochloric acid to adjust the pH to 6.1), ultrasonic for 30 minutes, filter, put the filtrate in a 50°C water bath to rotary evaporate to alcohol-free, add boiling water (conductivity 4.2μs / cm ), mix well, let cool and crystallize, take the crystals and add boiling water to dissolve them, absorb them with activated carbon, suction filter while they are hot, let the filtrate cool and crystallize, repeat several times, when the precipitated crystals are light yellow, take the crystals and add boiling water to dissolve them, absorb them with activated carbon, Filtrate, extract the filtrate with petroleum ether while it is hot, let the water layer cool and crystallize to obtain white crystals, filter, and dry to obtain the product. After testing, the optical purity (ee%) of the product was 98.2%.
Embodiment 3
[0090] Example 3 Extracting dihydromyricetin from vine tea
[0091] Take the stems and leaves of rattan tea, add 10 times the amount of 95% ethanol (adjust the pH to 4.6 with hydrochloric acid), ultrasonicate for 60 minutes, filter, put the filtrate in a 40°C water bath to rotary evaporate to alcohol-free, add boiling water (the water conductivity is 2.7μs / cm) and mix well, let cool and crystallize, take the crystals and add boiling water to dissolve them, absorb them with activated carbon, suction filter while they are hot, let the filtrate cool and crystallize, repeat several times, when the precipitated crystals are light yellow, take the crystals and add boiling water to dissolve, activated carbon Adsorption, filtration, the filtrate was extracted with petroleum ether while it was hot, and the water layer was cooled and crystallized to obtain white crystals, which were filtered and dried. After testing, the optical purity (ee%) of the product was 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com