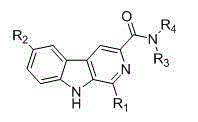
Harmaline amide compound as well as preparation method and application thereof
A technology of salsaline amide and compound, which is applied in the field of salsaline amide compounds and their preparation, can solve problems such as in-depth system derivation synthesis, structure-activity relationship research, and significant application significance, achieving significant antibacterial activity, Simple structure and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
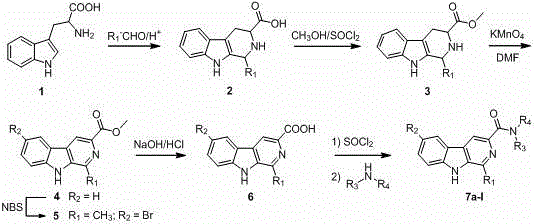
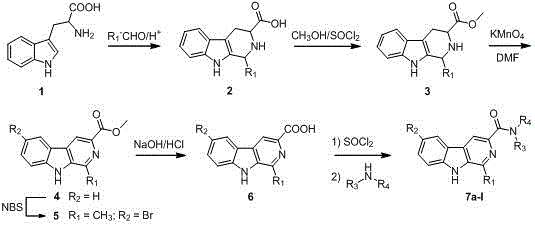
[0040] Example 1 Synthesis of N,N-diethyl-1-phenyl--β-carboline-3-amide (6a)
[0041] 1. Step A: Synthesis of 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2)
[0042] Weigh 20.4g of L-tryptophan (0.1mol) into a three-neck flask equipped with an electric heating mantle and electric stirrer, add 100mL of glacial acetic acid and 12.25g of benzaldehyde (0.12mol) while stirring, and stir at room temperature for 15min. Raise the temperature to 80-100°C and react for 10 hours. TLC traces until the tryptophan disappears. Stop heating. Cool to room temperature and a precipitate forms. Filter and concentrate the filtrate under reduced pressure to remove excess acetic acid and water to obtain a large number of light brown flakes. , and washed with water to obtain a white product, namely 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, with a yield of 85%.
[0043] 2. Step B: Synthesis of 1-phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (3)...
Embodiment 2
[0053] Example 2 Synthesis of N,N-diethyl-1-methyl-β-carboline-3-amide (7b)
[0054] 1, operation is the same as embodiment 1, only in step A replaces benzaldehyde with acetaldehyde.
[0055] 2. The product detection data are as follows: Yield: 84%; Melting point: 168-170°C; IR (KBr) ν: 3457, 2946, 1633; 1 H-NMR (500MHz, DMSO- d 6 ) δ: 1.19-1.22 (m, 6H, CH 3 ), 2.87 (s, 3H, 1-CH 3 ), 3.49-3.52 (m, J =12.5Hz, 4H, CH 2 ), 7.31 (t, J =15Hz, 1H, 6-H), 7.60 (dd, J =15Hz, 1H, 7-H), 7.67 (t,J =8.5Hz, 1H, 8-H), 8.37 (d, J =7.5Hz, 1H, 5-H), 8.48 (s, 1H, 4-H), 11.82 (s, 1H, 9-NH).
Embodiment 3
[0056] Example 3 Synthesis of N,N-diethyl-β-carboline-3-amide (7c)
[0057] 1, operation is the same as embodiment 1, only in step A replaces benzaldehyde with formaldehyde.
[0058] 2. The product detection data are as follows: Yield: 75%; Melting point: 174-176°C; IR (KBr) ν: 3167, 2977, 2928, 1593, 1096; 1 H-NMR (500MHz, DMSO- d 6 ) δ: 1.19-1.22 (m, 6H, CH 3 ), 3.49-3.51 (m, 4H, CH 2 ), 7.29 (t, J =14.5Hz, 1H, 6-H), 7.63 (dd, J =14.5Hz, 1H, 7-H), 7.73 (d, J =8.5Hz, 1H, 8-H), 8.39 (d, J =8.5Hz, 1H, 5-H), 8.49 (s, 1H, 4-H), 9.05 (s, 1H, 1-H), 11.79 (s, 1H, 9-NH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com