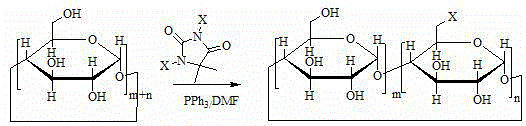
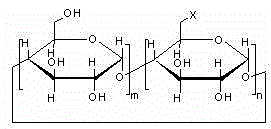
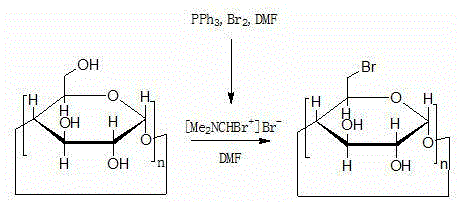
Method for preparing 6-deoxy-6-haloalkyl cyclodextrin
A cyclodextrin and halide technology, applied in the field of pharmaceutical preparation, can solve the problems of strong volatility, low substitution reaction yield, poor stability of succinimide and the like, achieve stable properties, improve reaction quality, and facilitate industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 6-perdeoxy-6-perchloro-α-cyclodextrin
[0030]
[0031] At room temperature, dissolve 50g of dry α-cyclodextrin in 1L of dry N,N-dimethylformamide, add 160g of triphenylphosphine, 1,3-dichloro-5,5-dimethylformamide in sequence Genhydantoin 100g, reacted at 70°C for 5h, dissolved 30g of sodium methoxide in 200mL of methanol, slowly added the solution into the reaction solution, slowly added the reaction solution into 18L of drinking water, a large amount of solid was precipitated, suction filtered, 100mL of cold water The filter cake was washed with N,N-dimethylformamide to obtain 37 g of off-white solid with a yield of 74% and a content of 95.8%.
[0032] The experimental data are as follows: MS (m / z): 1104.5 [M+Na]+. 1H-NMR (d6-DMSO): δ5.90-5.91 (16H, m), δ4.99-5.01 (8H, m), δ3.95-3.99 (8H, m), δ3.85-3.81 (8H, m), δ3.64-3.71 (8H, m), δ3.55-3.60 (8H, m), δ3.31-3.37 (16H, m).
Embodiment 2
[0034] Preparation of 6-perdeoxy-6-perbromo-β-cyclodextrin
[0035]
[0036] At room temperature, dissolve 10 g of dry β-cyclodextrin in 1 L of dry N,N-dimethylformamide, add 32.1 g of triphenylphosphine, 1,3-dibromo-5,5-di Methylhydantoin 17g, reacted at 80°C for 2.5h, dissolved 6.2g of sodium methoxide in 50mL of methanol, slowly added the solution into the reaction solution, slowly added the reaction solution into 10L of drinking water, a large amount of solids were precipitated, and suction filtered , 60mL of cold N,N-dimethylformamide washed the filter cake to obtain 8.3g of off-white solid with a yield of 83% and a content of 95.3%.
[0037] The experimental data are as follows: MS (m / z): 1576.5 [M+Na]+. 1H-NMR (d6-DMSO): δ5.88-5.90 (16H, m), δ4.91-5.04 (8H, m), δ3.91-3.93 (8H, m), δ3.84-3.80 (8H, m), δ3.61-3.72 (8H, m), δ3.53-3.62 (8H, m), δ3.27-3.33 (16H, m).
Embodiment 3
[0039] Preparation of 6-perdeoxy-6-periodo-γ-cyclodextrin
[0040]
[0041] At room temperature, dissolve 100g of dry γ-cyclodextrin in 2L of dry N,N-dimethylformamide, add 323.5g of triphenylphosphine, 1,3-diiodo-5,5-di Methylhydantoin 230g, reacted at 50°C for 4h, dissolved 75g of sodium methoxide in 500mL of methanol, slowly added the solution into the reaction solution, slowly added the reaction solution into 30L of drinking water, a large amount of solids were precipitated, suction filtered, 200mL The filter cake was washed with cold N,N-dimethylformamide to obtain 80 g of a khaki solid with a yield of 80% and a content of 97.8%.
[0042] The experimental data are as follows: MS (m / z): 2198.7 [M+Na]+. 1H-NMR (d6-DMSO): δ5.94-5.97 (16H, m), δ5.01-5.02 (8H, m), δ3.96-3.99 (8H, m), δ3.80-3.83 (8H, m), δ3.66-3.70 (8H, m), δ3.60-3.63 (8H, m), δ3.34-3.40 (16H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com