Phosphatidase C mutant and use thereof
A technology of phospholipase and application, which is applied in the fields of application, hydrolase, and fat production, and can solve the problems of less research on heterologous expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: Construction of wild-type BC-PC-PLC Pichia pastoris expression strain
[0099] According to the mature peptide sequence (PDB ID: 1AH7) of the phosphatidylcholine-specific phospholipase C of Bacillus cereus and the codon preference of Pichia pastoris, the DNA sequence of BC-PC-PLC (SEQ ID No: 1) was designed ), and at its front fusion α factor signal peptide sequence (its DNA sequence is derived from commercial Pichia pastoris expression vector pPIC-9k, the 8th-274th in SEQ ID No:3) and the Kozak sequence of Pichia pastoris (SEQ 1-7 in ID No:3), and finally obtained the α-BC-PC-PLC DNA sequence (SEQ ID No:3).
[0100] The α-BC-PC-PLC DNA sequence was provided to Shanghai Sangon Biotechnology Co., Ltd. for whole gene synthesis, and the cloning vector pGEM-T-PLC containing the α-BC-PC-PLC DNA sequence was obtained. Using this vector as a template, use HS DNA polymerase and primer pair AmPLC-3 / AmPLC-4 were used to amplify the PLC fragment by PCR.
[0101] Usi...
Embodiment 2
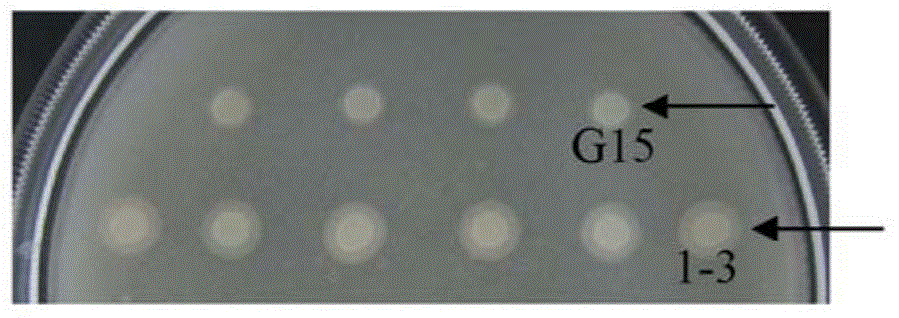
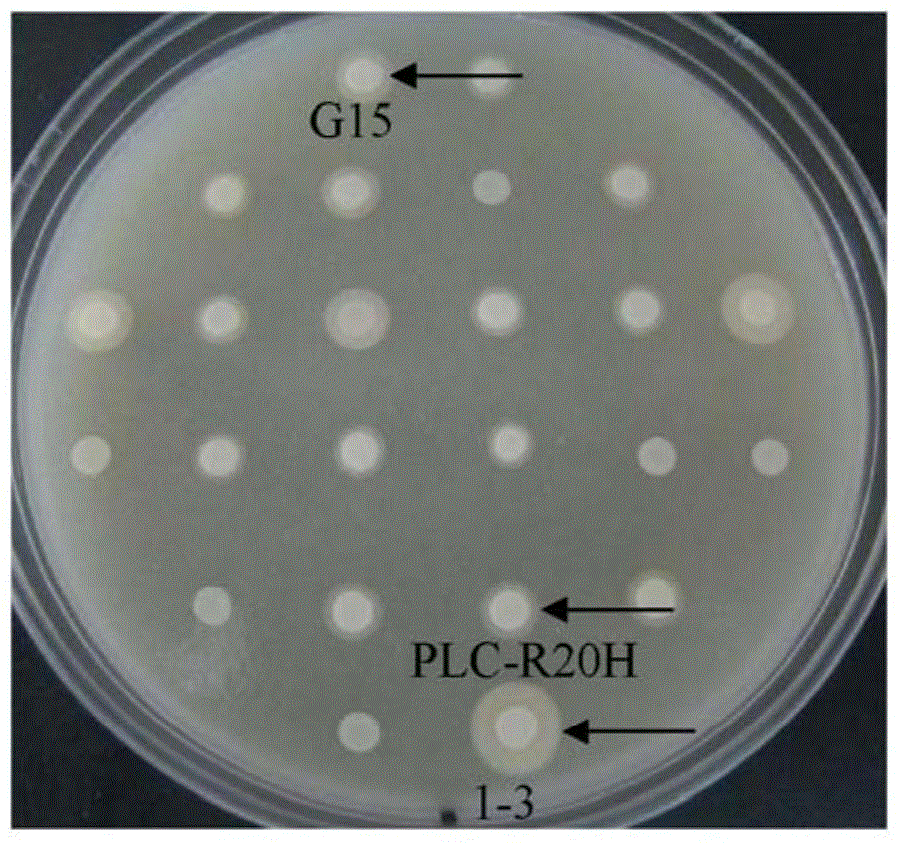
[0105] Example 2: Construction and screening of BC-PC-PLC mutant library
[0106] Using the pAO-PLC vector as a template, use HS DNA polymerase and primer pair AmPLC-1 / AOXH-2, a fragment of about 900bp was amplified by PCR. Using the pAO-PLC vector as a template, use HS DNA polymerase and primer pair AOXH-3 / AmPLC-4, amplify about 1.1kb fragment by PCR, mix about 900bp fragment and about 1.1kb fragment obtained by the previous two steps of PCR as the template of the third step PCR, use the primer pair AmPLC-1 / AmPLC-4 and HS DNA polymerase, a fragment of about 1.9kb was amplified by PCR.
[0107] The about 1.9kb fragment was cloned into pAO-PLC through AatII and EcoRI restriction sites to obtain pmAO-PLC. In pmAO-PLC, a HindIII restriction site in pAO-PLC was mutated so that only a HindIII restriction site located at the 5' end of the BC-PC-PLC sequence was retained, so that BC could be converted to BC using HindIII and EcoRI - The mutated fragment of PC-PLC was cloned i...
Embodiment 3
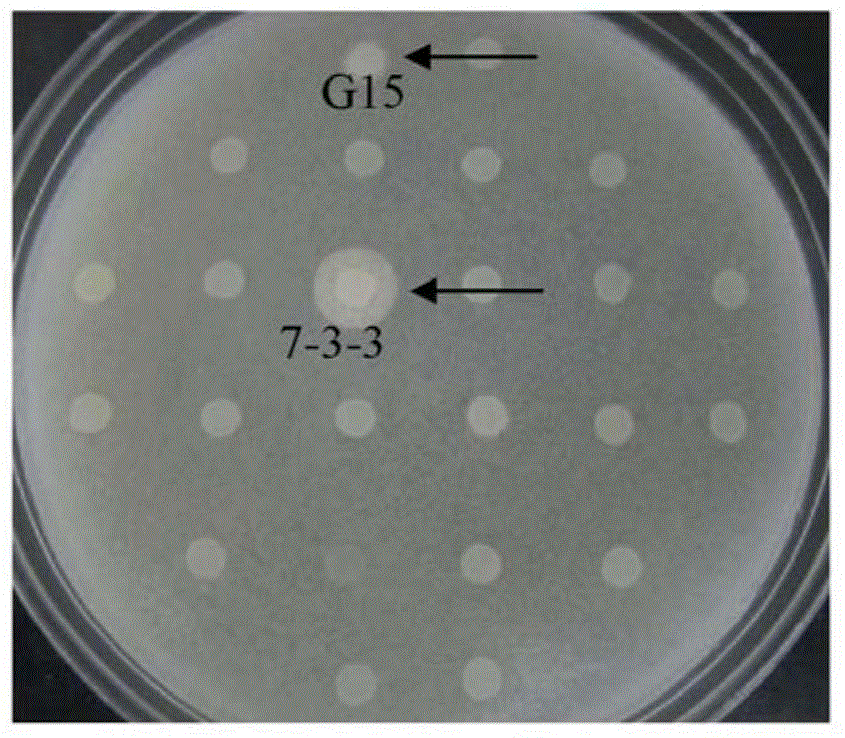
[0110] Embodiment 3: BC-PC-PLC mutant sequence analysis
[0111] The 7-3-3 strain was inoculated in 3ml YPD liquid medium, cultivated overnight at 30°C, and the genomic DNA was extracted. Using the genomic DNA of the 7-3-3 strain as a template, use HS DNA polymerase and primers were used for PCR amplification of AOX1-5 / AOX1-3 to obtain the DNA sequence of BC-PC-PLC in the 7-3-3 strain. The obtained sequence was sent to Shanghai Sangon Bioengineering Co., Ltd., and AOX1-5 / AOX1-3 was sequenced with primers. The DNA sequencing result of 7-3-3 BC-PC-PLC is shown in SEQ ID No:4. After comparison, it was found that compared with SEQ ID No: 3, there were 7 base mutations in SEQ ID No: 4, including three sense mutations, which were the mutation of G at position 59 to A, making the Arginine at position 20 is mutated to histidine (CGT→CAT); A at position 188 is mutated to G, asparagine at position 63 is mutated to serine (AAC→AGC); C at position 248 is mutated to A, making Alanine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com