Chitin deacetylase, and construction method and application thereof
A deacetylase and construction method technology, applied in the direction of microorganism-based methods, applications, botany equipment and methods, etc., can solve the problems of uniformity, high alkali consumption, and inability to obtain quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Cloning of Bacillus cereus chitin deacetylase gene
[0026] Primers were designed to amplify the chitin deacetylase (CDA) of B. cereus ATCC10987 based on the sequence information annotated as chitin deacetylase (CDA) in 13 B. cereus genome sequences provided by NCBI Gene. Primers are:
[0027] BcCDA-dF: ATGTTTTTCTTTTTTTATTACGAG
[0028] BcCDA-dR: TTATTGAACATCTTTACTTTTCG
[0029] B. cereus ATCC10987 was used as a template.
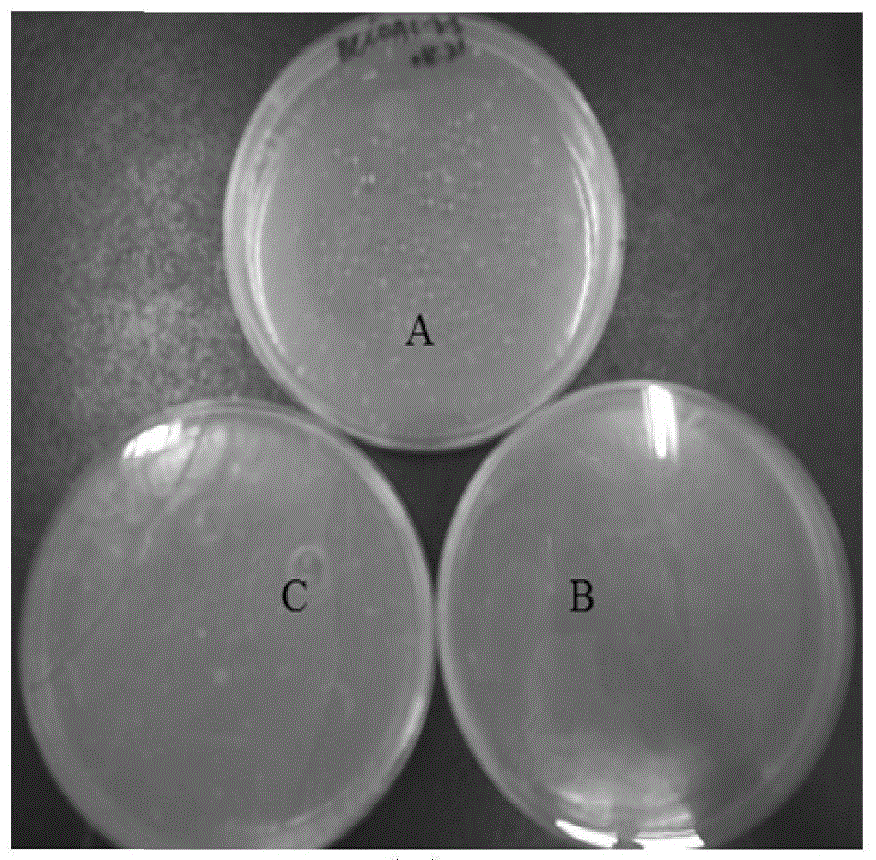
[0030] The PCR reaction system is: template 1 μL; 5 μL 10×PCR buffer, 2 μL dNTP (10 mmol / L), 2 μL primer BcCDA-dF (10 μmol / L), 2 μL primer BcCDA-dR (10 μmol / L), 1 μL Taq DNA polymerase ( 5U / μL), add ddH2O to make up to 50 μL. PCR reaction conditions: pre-denaturation at 95°C for 5min; denaturation at 95°C for 30s, annealing at 55°C for 30s, extension at 72°C for 2min, 30 cycles; extension at 72°C for 10min. Use 1% agarose gel electrophoresis to perform electrophoresis analysis on the PCR amplification products, cut the gel of the PCR...
Embodiment 2
[0050] Embodiment 2: In vitro recombinant expression, isolation and purification and biological activity analysis of Bacillus cereus BcCDA gene.
[0051] In vitro recombinant construction method:
[0052] 1. Construct chitin deacetylase recombinant expression vector;
[0053] 2. Introducing the recombinant expression vector obtained in step 1) into the Escherichia coli host cell, performing induced expression, and obtaining the expression product;
[0054] 3. Separation and purification of the recombinant protein obtained in step 2), namely chitin deacetylase.
[0055] In step 1), the expression vector can be pCT7-CHISP6H.
[0056] In step 2), the host cell is Escherichia coli BL21(DE3).
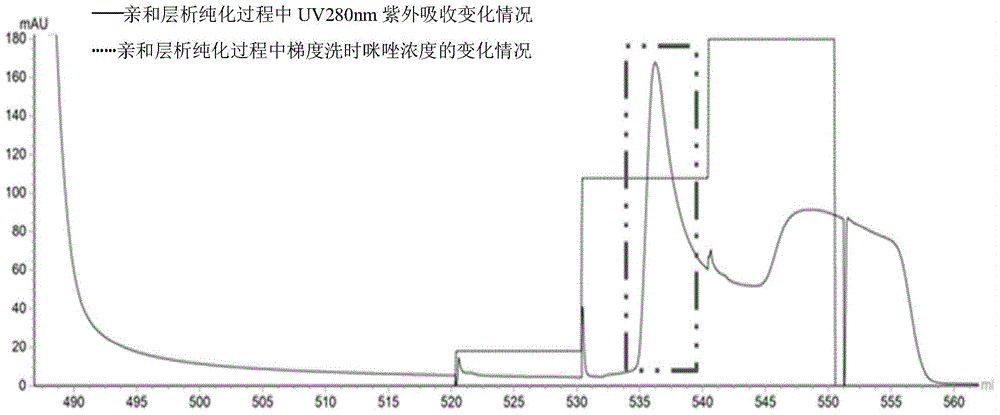
[0057] In step 3), the separation and purification method can be metal ion affinity chromatography
[0058] Construction of recombinant expression vector pCT7-CHISP6H-BcCDA
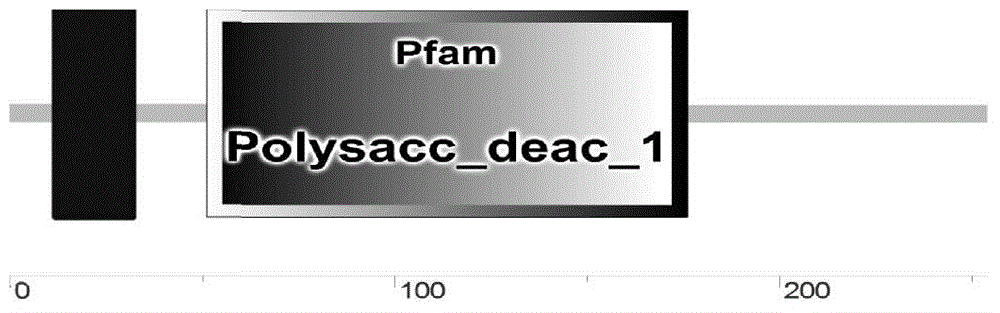
[0059] After online analysis (http: / / smart.embl-heidelberg.de / ) of the deduced amino acid sequence characteristi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com