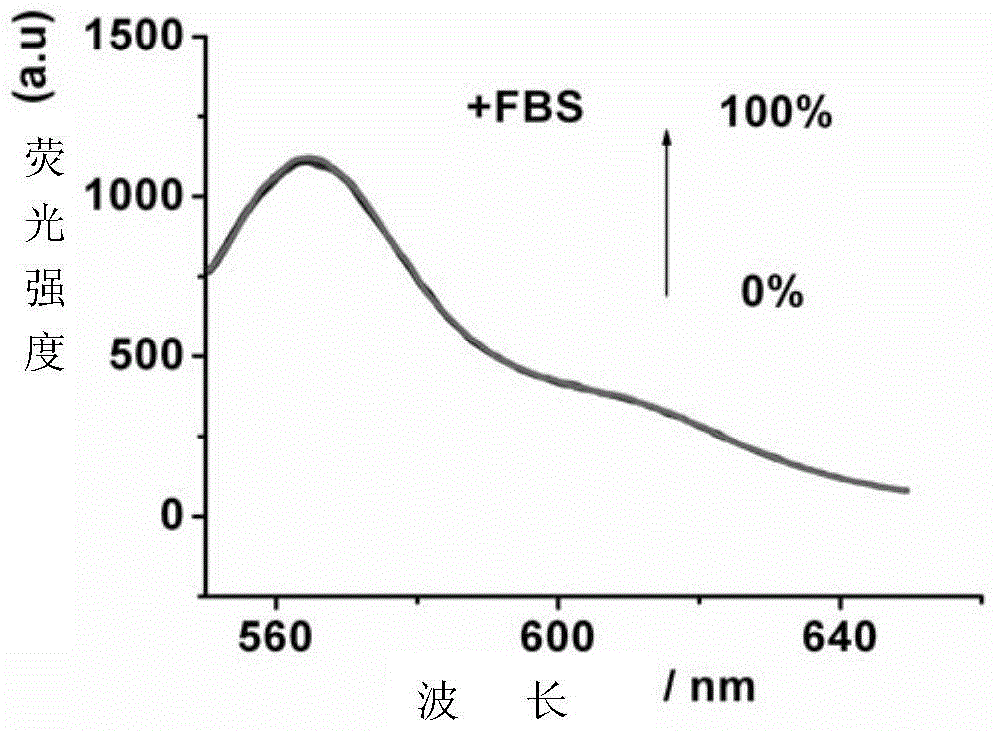
N, n-dipentane substituted quinacridone compounds, preparation method and application thereof
A technology for compounds and identification methods, applied in the field of medicine, can solve problems such as limitations, and achieve the effects of real-time on-the-spot detection, low cost, and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
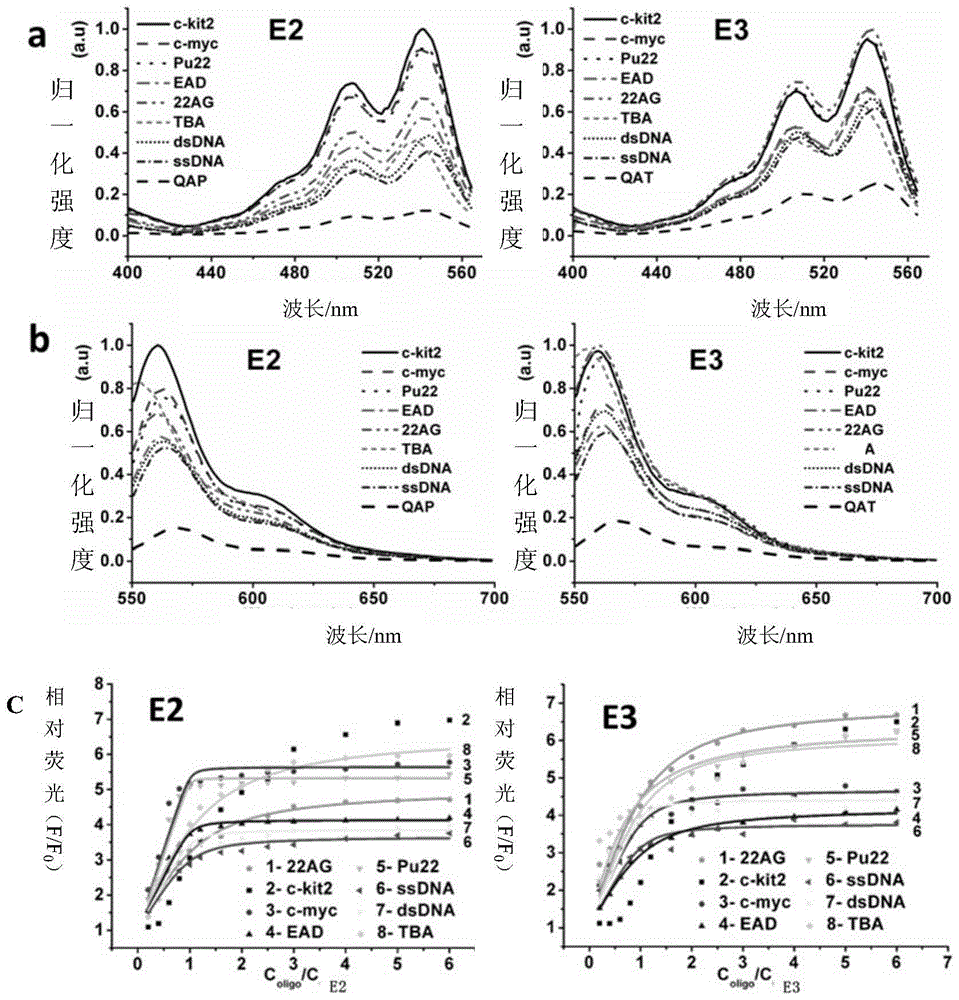
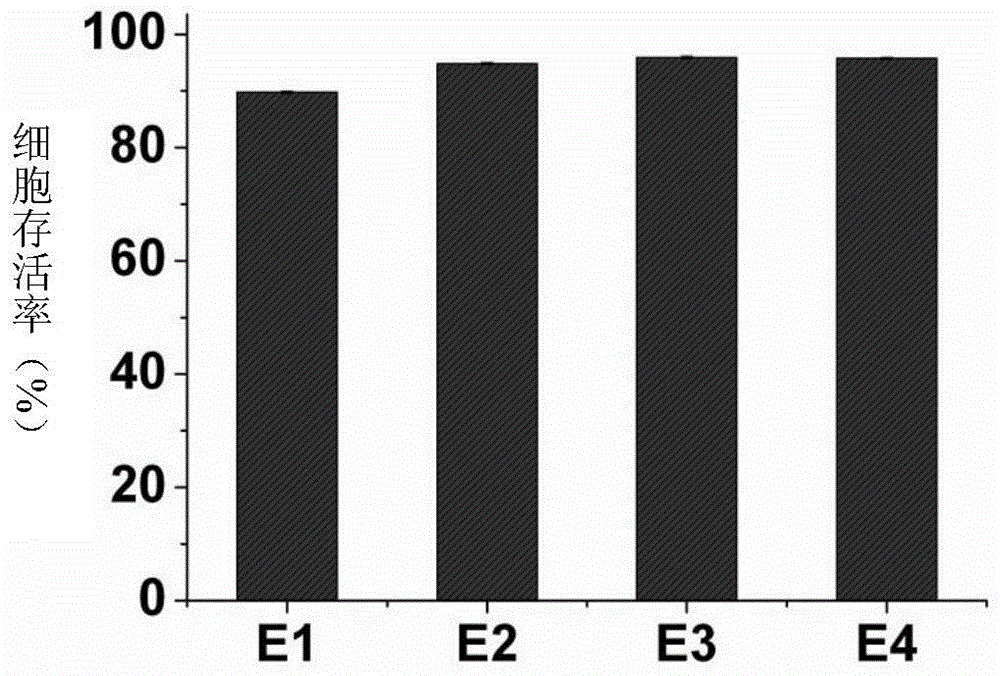
[0065] Embodiment 1, the synthesis of compound E1 (QAB)
[0066] Dissolve NaH (200mg, 60%) and quinacridone (624mg, 2mmol) in dry THF (10mL), heat to reflux for 1h, add 5mg of tetrabutylammonium bromide, 1,5 di Pentyl bromide (2.04 g, 10 mmol) was refluxed overnight. Post-treatment: After the reaction solution was allowed to cool, 5 mL of methanol was added to quench the reaction. After the solvent was evaporated, solid column chromatography was used for separation (washing away impurities with dichloromethane of 2 times the column volume, and then using dichloromethane with a volume ratio of 1:1) Eluted with methanol and collected the eluate) to obtain compound E1. The yield was 41%.
[0067] The confirmed data of the compound structure are: 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 8.72 (d, J = 9.0Hz, 2H), 8.55 (dd, J = 1.8Hz, J = 8.1Hz, 2H), 7.78-7.72 (m, 2H), 7.48 (d, J = 8.7Hz, 2H), 7.27(t, J=7.5Hz, 3H), 4.52(t, J=7.8Hz, 4H), 3.49(t, J=6.6Hz, 4H), 2.10-1.99(m, 8H) ,1.85-1....
Embodiment 2
[0068] Embodiment 2, the synthesis of compound E2 (QAP)
[0069] Basically the same as Example 1, except that 10 mmol of compound 1-(5-bromopentyl)-4-methylpiperazine was used instead of 1,5-dibromopentane, and the reaction was refluxed overnight. After evaporating the solvent, solid column chromatography (first wash away impurities with 2 times column volume of dichloromethane, then elute with dichloromethane and methanol at a volume ratio of 1:1, and collect the eluent) to obtain compound E2, The yield was 71%.
[0070] The confirmed data of the compound structure are: 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 8.73 (s, 2H), 8.55 (dd, J = 1.2Hz, J = 8.1Hz, 2H), 7.76-7.71 (m, 2H), 7.48 (d, J = 8.7Hz, 2H) ,7.26(t,J=7.5Hz,3H),4.50(t,J=7.8Hz,4H),2.47-2.39(m,18H),2.29(s,7H),2.02(s,7H),1.65( t,J=3.3Hz,8H); 13 C NMR (100MHz, CDCl 3 ):δ(ppm)177.8,142.0,135.4,134.5,127.9,126.0,120.9,120.8,114.6,113.3,58.3,55.0,53.0,46.1,45.9,26.9,26.5,24.9.ESI HRMS exact masscalcd.for C 40 h 53 o 2 ...
Embodiment 3
[0071] Embodiment 3, the synthesis of compound E3 (QAT)
[0072] It is basically the same as Example 1, except that 10 mmol of the compound 5-bromo-N,N-dimethylpentanyl-1-amine is used instead of 1,5 dibromopentane, the reaction is refluxed overnight, and the solvent is evaporated Afterwards, solid column chromatography separation (first wash away impurities with dichloromethane of 2 times of column volume, then elute with volume ratio 1:1 dichloromethane and methanol, collect eluent), obtain compound E3, yield is 42%.
[0073] The confirmed data of the compound structure are: 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 8.72 (s, 2H), 8.54 (dd, J = 1.2Hz, J = 7.8Hz, 2H), 7.75-7.70 (m, 2H), 7.48 (d, J = 9.0Hz, 2H) ,7.27-7.22(m,3H),4.50(t,J=7.8Hz,4H),2.36(t,J=6.3Hz,4H),2.25(t,J=2.4Hz,15H),2.03(d, J=8.1Hz, 4H), 1.65(s, 8H); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 178.0, 142.1, 135.5, 134.6, 128.0, 126.1, 121.0, 120.8, 114.6, 113.4, 59.7, 46.3, 45.6, 27.5, 27.1, 24.9.ESI HRMS exact massca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com