Praseodymium-holmium-codoped rare earth stannate up-conversion luminescent material and its preparation method and use
A technology of luminescent materials and co-doping, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the above-mentioned praseodymium-holmium co-doped rare earth stannate up-conversion luminescent material comprises the following steps;
[0031] Step S101, according to the chemical formula Me 2-x-y SnO 5 :xPr 3+ ,yHo 3+ The stoichiometric ratio of each element in is weighed as Me 2 o 3 , SnO 2 、Pr 2 o 3and Ho 2 o 3 Powder, wherein, x is 0.002-0.06, y is 0.002-0.04, and the Me 2 o 3 It is yttrium oxide, lanthanum oxide, gadolinium oxide or lutetium oxide.
[0032] Preferably, x is 0.03 and y is 0.01.
[0033] The Me 2 o 3 , SnO 2 、Pr 2 o 3 and Ho 2 o 3 The powder molar ratio is (2-x-y): 2:x:y.
[0034] Step S102, dissolving the weighed powder in nitric acid to prepare a mixed solution; the Me 2 o 3 , SnO 2 、Pr 2 o 3 and Ho 2 o 3 The total concentration of the powder in the mixed solution is 0.5mol / L-3mol / L; then a dispersant is added to the mixed solution to obtain a precursor solution;
[0035] Preferably, the Me 2...
Embodiment 1
[0050] Weigh Y respectively according to the ratio of 1.96:2:0.03:0.01 in molar ratio 2 o 3 , SnO 2 , Pr 2 o 3 and Ho 2 o 3 The powder is dissolved in nitric acid to prepare a 1.5mol / L mixed solution, and polyethylene glycol is added as an additive to obtain a precursor solution; the concentration of polyethylene glycol in the precursor solution is 0.01mol / L. Then put the precursor solution into the atomization device, and then feed 5 L / min argon into the atomization device. The precursor solution enters a quartz tube with an inlet temperature of 180°C and an outlet temperature of 110°C along with the argon carrier gas to generate a precursor. The diameter of the quartz tube is 30mm and the length is 3m. The precursor is collected and placed in a temperature-programmed furnace for calcination for 3 hours, the calcination temperature is 1100°C, and the chemical formula is Y 1.96 SnO 5 : 0.03Pr 3+ , 0.01Ho 3+ up-converting luminescent materials.
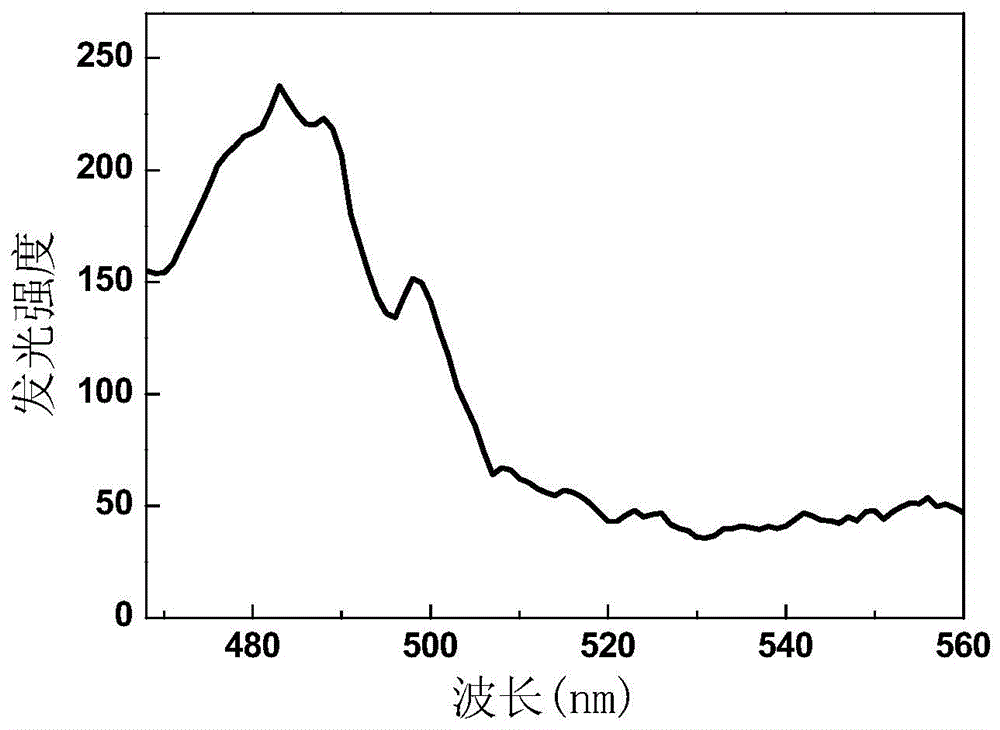
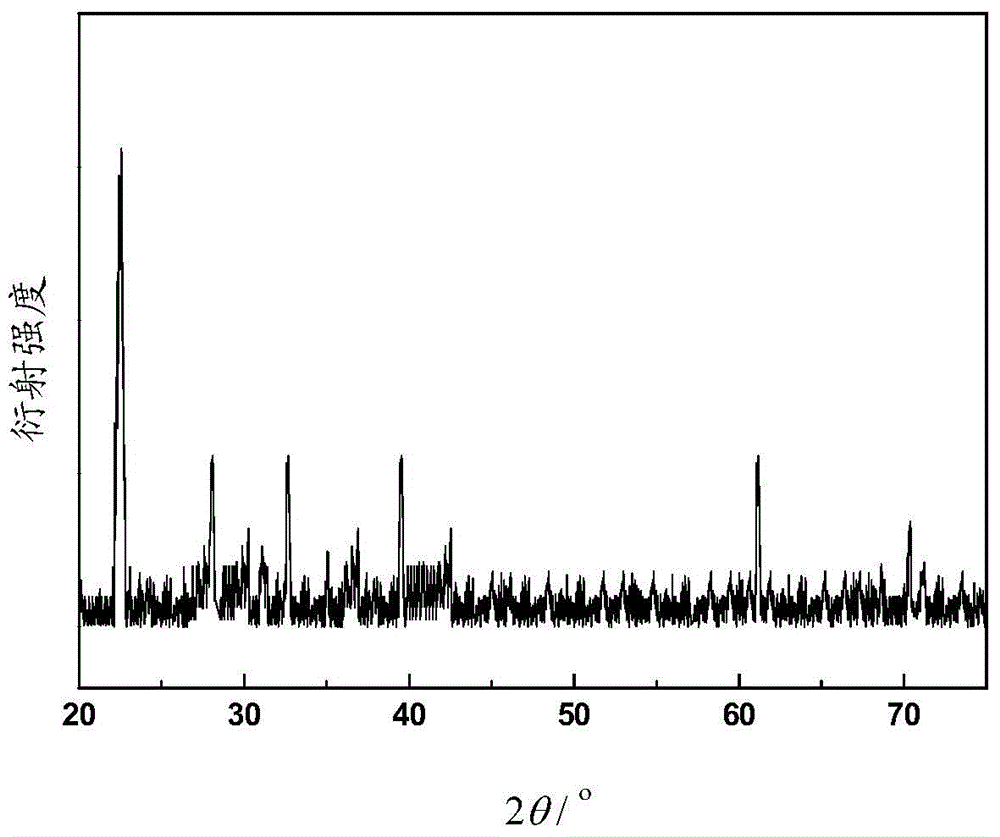
[0051] see figure ...
Embodiment 2
[0056] Weigh Y respectively according to the ratio of 1.9:2:0.06:0.04 in molar ratio 2 o 3 , SnO, Pr 2 o 3 and Ho 2 o 3 The powder was dissolved in nitric acid to prepare a mixed solution of 3 mol / L, and polyethylene glycol was added as an additive to obtain a precursor solution; the concentration of polyethylene glycol in the precursor solution was 0.05 mol / L. Then put the precursor solution into the atomization device, and then feed 15 L / min argon gas into the atomization device. The precursor solution enters a quartz tube with an inlet temperature of 220°C and an outlet temperature of 130°C along with the argon carrier gas to form a precursor. The diameter of the quartz tube is 150mm and the length is 0.5m. The precursor is collected and placed in a temperature-programmed furnace for calcination. After 5 hours, the calcination temperature is 1300°C, and the chemical formula is Y 1.9 SnO 5 : 0.06Pr 3+ , 0.04Ho 3+ Up-converting luminescent material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com