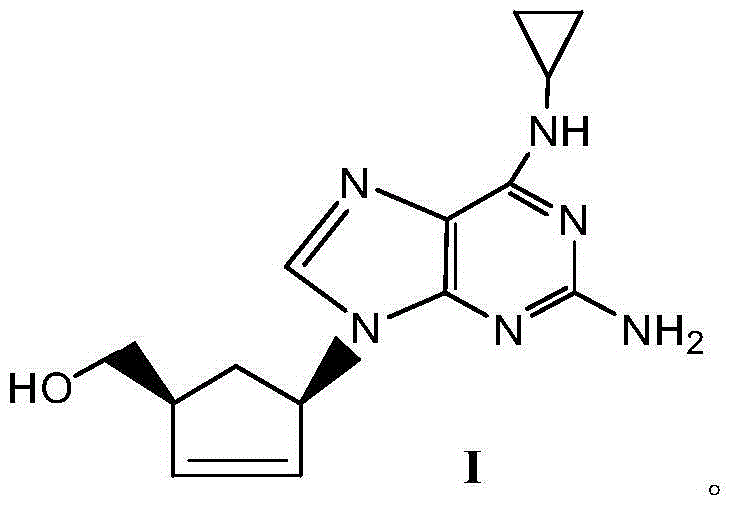
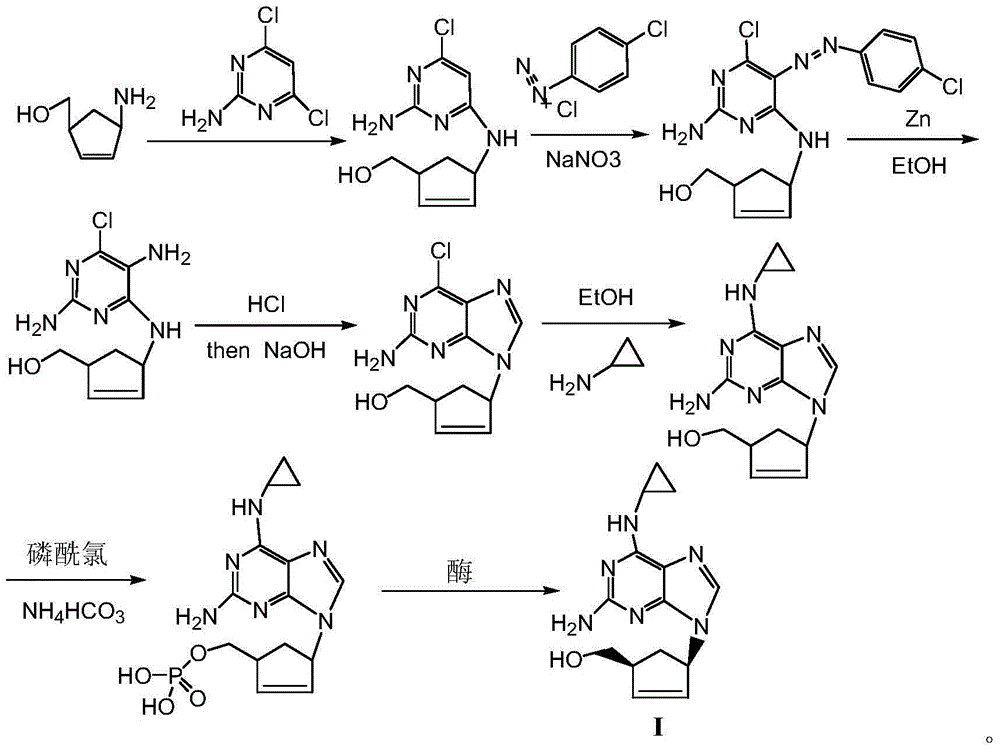
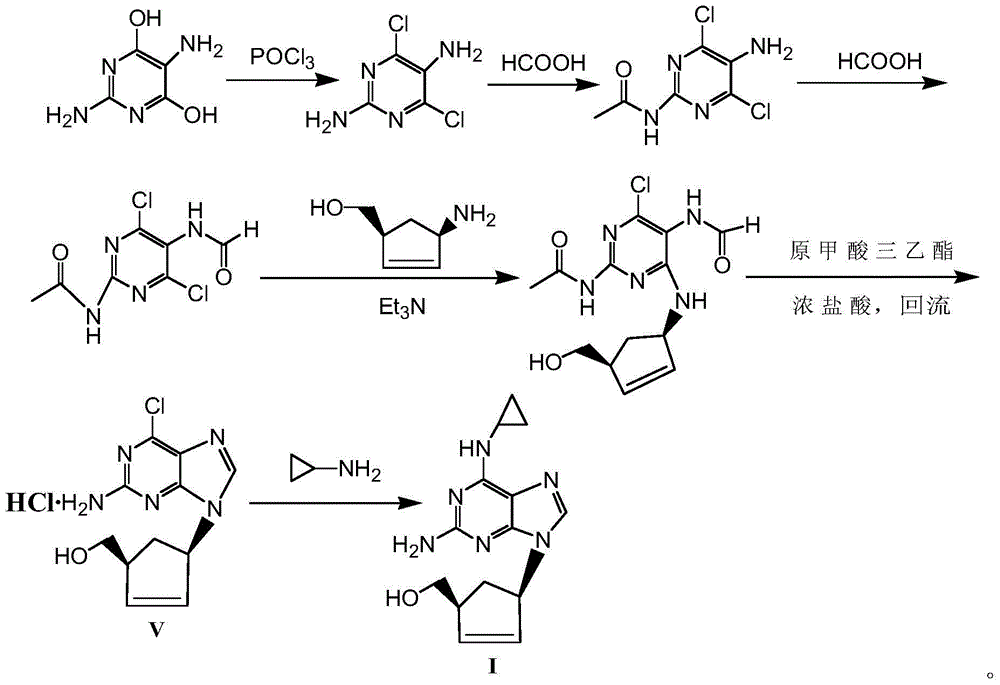
Process for preparing abacavir intermediate in formula V by adopting one-pot method
A technology for abacavir and intermediates, applied in the field of preparation of abacavir formula V intermediates, can solve problems such as failure to meet industrialization requirements, easy formation of by-products, poor product quality, etc., to facilitate large-scale production and yield The effect of high efficiency and simplified preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The formula II compound (53.2g, 0.36mol) was added to 700mL of absolute ethanol, then sodium bicarbonate (100g, 0.94mol) was added, and stirred at room temperature for 0.5 hours under argon protection; the formula III compound (70g, 0.34 mol) into the above reaction system, heat up to 80°C and then keep warm for reaction. When TLC (PE / EA=1 / 1, V / V) detects that the reaction is basically complete (after about 6-7 hours of reaction), cool down to room temperature, Suction filtration; triethyl orthoformate (251.5 g, 1.69 mol) was added to the filtrate, and HCl-isopropanol solution (8.5 mol / L, 60 mL) was added dropwise to the reaction system under stirring at room temperature. Stir the reaction, when TLC (CH 2 Cl 2 / MeOH=10 / 1, V / V) detect that the reaction is complete (after about 6-7 hours of reaction), cool down to 10-15°C, and continue to stir for 0.5 hours; After vacuum drying at °C, 92.4 g of a light yellow to off-white intermediate of formula V was obtained, with a m...
Embodiment 2
[0035] The formula II compound (59.6g, 0.40mol) was added in 750mL isopropanol, then potassium bicarbonate (114.0g, 1.14mol) was added, and stirred at room temperature for 0.5 hours under argon protection; the formula III compound (78.3g ,0.38mol) into the above reaction system, heat up to 80°C and then keep warm for reaction. When TLC (PE / EA=1 / 1, V / V) detects that the reaction is basically complete (after about 6-7 hours of reaction), drop to Suction filtration at room temperature; triethyl orthoformate (450.5 g, 3.04 mol) was added to the filtrate, and HCl-ethanol solution (6.0 mol / L, 80 mL) was added dropwise to the reaction system under stirring at room temperature. Stir the reaction, when TLC (CH 2 Cl 2 / MeOH=10 / 1, V / V) detect that the reaction is complete (after about 6-7 hours of reaction), cool down to 10-15°C, and continue to stir for 0.5 hours; After vacuum drying at °C, 101.0 g of a light yellow to off-white intermediate of formula V was obtained, with a molar yie...
Embodiment 3
[0037] The formula II compound (32.8g, 0.22mol) was added in 750mL isopropanol, then triethylamine (83.6mL, 0.60mol) was added, under the protection of argon, stirred at room temperature for 0.5 hours; the formula III compound (41.2g ,0.20mol) into the above reaction system, heat up to 80°C and then keep warm for reaction. When TLC (PE / EA=1 / 1, V / V) detects that the reaction is basically complete (after about 6-7 hours of reaction), drop to Suction filtration at room temperature; triethyl orthoformate (118.6 g, 0.80 mol) was added to the filtrate, and HCl-ethanol solution (12.0 mol / L, 30 mL) was added dropwise to the reaction system under stirring at room temperature. Stir the reaction, when TLC (CH 2 Cl 2 / MeOH=10 / 1, V / V) detect that the reaction is complete (after about 6-7 hours of reaction), cool down to 10-15°C, and continue to stir for 0.5 hours; After vacuum drying at °C, 52.6 g of a light yellow to off-white intermediate of formula V was obtained, with a molar yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com