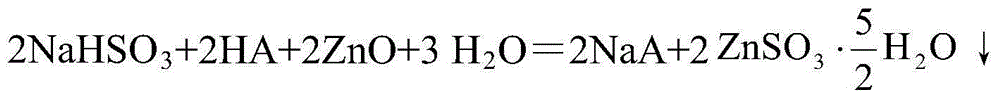Zinc oxide desulfurization process
A desulfurization process, zinc oxide technology, applied in chemical instruments and methods, dispersed particle separation, separation methods, etc., can solve problems such as pipeline blockage, circulation pump and pipeline wear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A zinc oxide desulfurization process is characterized in that: its preparation steps are:
[0029] Absorption process: The aqueous solution of sodium acetate (NaAc) is used as the absorption liquid. When the pH value is greater than or equal to 3.5, it is in reverse contact with the flue gas containing sulfur dioxide in the absorption tower. The sodium acetate in the absorption liquid reacts with the sulfur dioxide in the flue gas to generate acetic acid. and sodium bisulfite. Sodium acetate is the medium;
[0030] Reaction process: when the pH value is less than 3.5, the aqueous solution of the medium enters the reactor outside the absorption tower, reacts with zinc oxide, and regenerates NaAc and H in step (1). 2 O and precipitation;
[0031] Filtration: when the pH value was 6.5~9, the product in step (2) was filtered, and the filter residue was precipitation, the filtrate is an aqueous solution of NaAc;
[0032] Filtrate circulation: The filtrate in step (3) ...
Embodiment 2
[0034] A zinc oxide desulfurization process is characterized in that: its preparation steps are:
[0035] Absorption process: The aqueous solution of sodium hydroxide (NaOH) is used as the absorption liquid, and when the pH value is greater than or equal to 3.5, it is in reverse contact with the flue gas containing sulfur dioxide in the absorption tower, and the sodium hydroxide in the absorption liquid reacts with the sulfur dioxide in the flue gas. Sodium sulfite is generated, and sodium sulfite further reacts with sulfur dioxide in the flue gas to generate sodium bisulfite. The medium is sodium sulfite;
[0036] Reaction process: when the pH value is less than 3.5, the aqueous solution of the medium enters the reactor outside the absorption tower, reacts with zinc oxide, and regenerates Na in step (1). 2 SO 3 、H 2 O and precipitation;
[0037] Filtration: when the pH value was 6.5~9, the product in step (2) was filtered, and the filter residue was precipitated, the ...
Embodiment 3
[0041] A zinc oxide desulfurization process is characterized in that: its preparation steps are:
[0042] Absorption process: with sodium carbonate (Na 2 CO 3) is the absorption liquid, and when the pH value is greater than or equal to 3.5, it is in reverse contact with the flue gas containing sulfur dioxide in the absorption tower, and the sodium carbonate in the absorption liquid reacts with the sulfur dioxide in the flue gas to form sodium sulfite, which further reacts with the sulfur dioxide in the flue gas Sulfur dioxide reacts to form sodium bisulfite. The medium is sodium sulfite;
[0043] Reaction process: when the pH value is less than 3.5, the aqueous solution of the medium enters the reactor outside the absorption tower, reacts with zinc oxide, and regenerates Na in step (1). 2 SO 3 、H 2 O and ZnSO 3 precipitation;
[0044] Filtration: when the pH value was 6.5~9, the product in step (2) was filtered, and the filter residue was precipitated, the filtrate wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com