Method for separating isobutanol and ethyl isobutyrate azeotrope by continuous extractive distillation
A technology of ethyl isobutyrate and extractive distillation, which is applied in extractive distillation, chemical instruments and methods, preparation of carboxylate, etc., can solve problems such as unseen data, and achieve the effect of reducing cost, saving cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
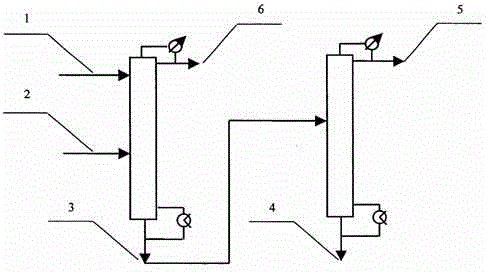
[0024] A method for continuous extraction and rectification separation of isobutanol and ethyl isobutyrate azeotrope, its process flow diagram is as follows figure 1 shown. The number of theoretical plates in the extractive distillation column 3 is 55. Use 95wt% ethylene glycol and 5wt% [Emim][OAc] as the extractant, add it from the third theoretical plate (the number of plates is from top to bottom, nozzle 1), and the flow rate is 1.8kg / hr. The mixture of isobutanol and ethyl isobutyrate is added from the 42nd theoretical plate (pipe port 2), the flow rate is 1kg / hr, the mass fraction of isobutyric acid is 52%, 3 is operated under normal pressure, and the tower top The reflux ratio is 7:1, the temperature at the top of the tower is 110~115°C, and the temperature at the bottom of the tower is 140~150°C. The product at the top of the tower is analyzed by gas chromatography, and the ethyl isobutyrate product with a purity higher than 99.8% is obtained.
[0025] The bottom of t...
Embodiment 2
[0027] The two-column process flow of the extractive distillation column goes through Example 1. 85wt% ethylene glycol and 15wt% [Emim][OAc] were used as the extractant, fed from the third theoretical plate, and the flow rate was 2.2kg / hr. The mixture of isobutanol and ethyl isobutyrate is added from the 42nd theoretical plate, the flow rate is 1kg / hr, and the composition is the same as in Example 1. The rectification column 3 operates under normal pressure with a reflux ratio of 5.0:1. At this time, the temperature at the top of the tower is 110~113°C, and the temperature at the bottom of the tower is 130~140°C. The overhead product is analyzed by gas chromatography to obtain an ethyl isobutyrate product with a purity higher than 99.7%.
[0028] The operating conditions of the solvent recovery tower are the same as in Example 1, and the bottom of the tower is returned to the extractive distillation tower 3 for recycling, and other conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com