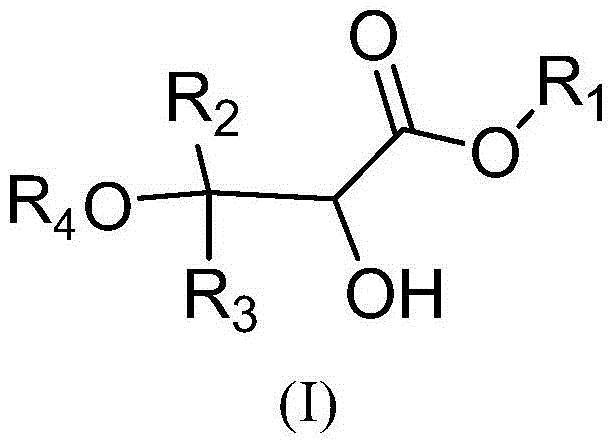
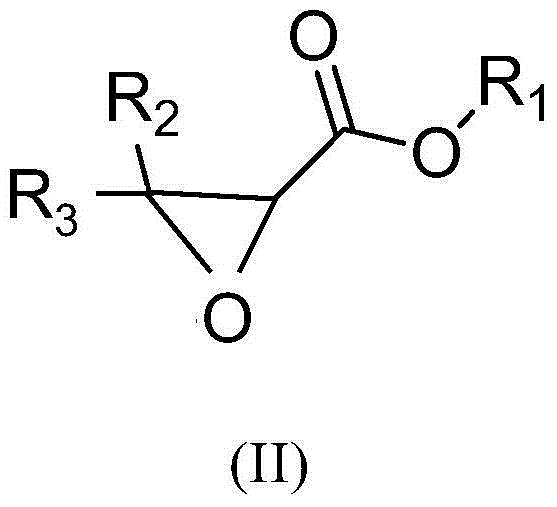
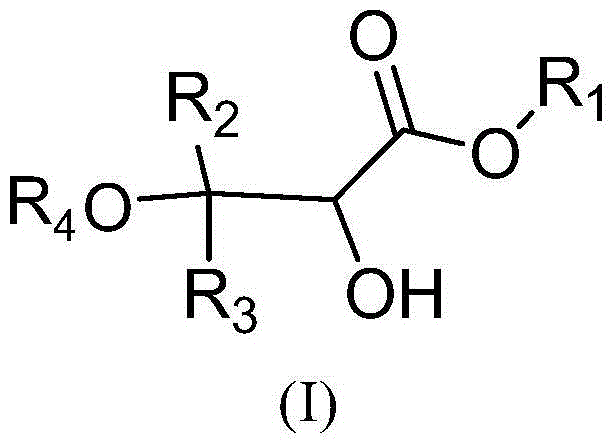
Method for synthesizing 2-hydroxy-3-alkoxy propionate compounds
A compound, methyl technology, applied in the synthesis of 2-hydroxy-3-alkoxy propionate compounds, the field of endothelin receptor inhibitor intermediates, can solve problems such as discomfort, toxic irritation and strong corrosiveness , to achieve a wide range of applications, easy industrial production, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Dissolve 600 grams of methyl 3,3-diphenyl-2,3-epoxypropionate in 2.4 liters of anhydrous isopropanol, stir at 20°C, slowly add 48 grams of hydrochloric acid, keep the system temperature after adding After stirring for 1 hour at 20°C, add 4.8 liters of water, continue to stir for half an hour, filter with suction, separate the solid product, and dry it in vacuum at 50°C to obtain 2-hydroxy-3-isopropoxy-3,3-diphenyl methyl propionate.
[0035] Yield: 95.5%
[0036] HPLC purity: 99.7%
Embodiment 2
[0038] Dissolve 10 kg of ethyl 3,3-diphenyl-2,3-epoxypropionate in 120 liters of anhydrous methanol, stir at 25°C, slowly add 2 kg of hydrochloric acid, and keep the system temperature at 30 ℃, stirred for 1 hour, added 150 liters of water, stirred for 2 hours, discharged, filtered, and the filter cake was vacuum-dried at 50°C to obtain 2-hydroxy-3-methoxy-3,3-diphenylpropionic acid ethyl ester.
[0039] Yield: 96.2%
[0040] HPLC purity: 99.3%
Embodiment 3
[0042] Dissolve 450 grams of 3,3-diphenyl-2,3-glycidylpropionate in 2.7 liters of absolute ethanol, stir at 30°C, slowly add 80 grams of hydrochloric acid, after the addition is complete, keep the temperature at 40°C After stirring for 1.5 hours, add 3 liters of water, stir for 0.5 hours, filter with suction, and dry the filter cake under vacuum at 50°C to obtain propyl 2-hydroxy-3-ethoxy-3,3-diphenylpropionate.
[0043] Yield: 95.7
[0044] HPLC purity: 99.1%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com