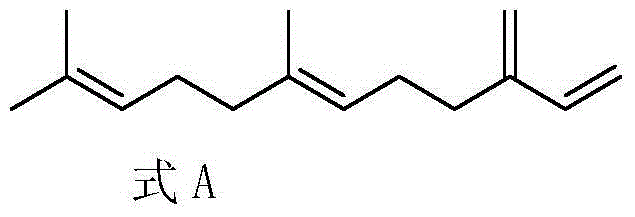
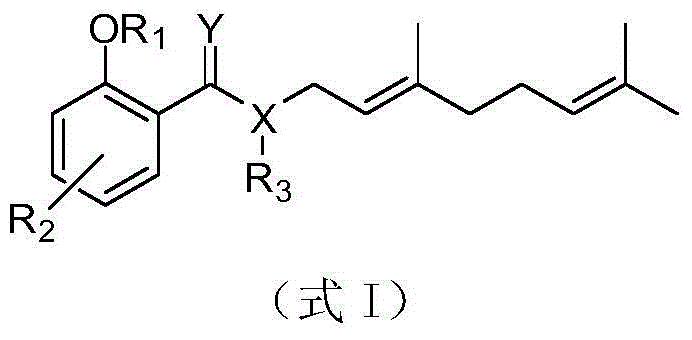
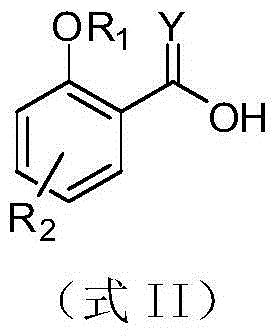
Salicylic acid trans-(beta)-farnesene analogues and application thereof
A technology of farnesene and its analogues, which is applied in the field of salicylic acid trans-farnesene analogues and their preparation, which can solve the problems of limiting the application of EBF, unstable properties, and easy oxidation, and achieves a simple and convenient preparation method Ease of operation, low cost, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of 3-methoxysalicylic acid-(E)-3,7-dimethyl-2,6-octadienyl ester (I-10)
[0037] In a 100ml three-neck flask, add 2.18g 3-methoxysalicylic acid, 3.20g DCC, 1.74g DMAP and 20mL THF, add dropwise 2.00g geraniol, and react at 20°C for 3 hours. Extract with water three times, take the organic phase, remove the solvent under reduced pressure, and separate by silica gel column chromatography (eluent is petroleum ether:ethyl acetate V:V=500:1) to obtain yellow liquid 3-methoxysalicylic acid -(E)-3,7-dimethyl-2,6-octadienyl ester (I-10), yield 53.8%. 1H NMR: 11.10 (s, 1H, ArOH), 7.26-7.47 (m, 1H, ArH), 7.02-7.09 (m, 1H, ArH), 6.81 (t, 1H, J=8.07Hz, ArH), 5.43- 5.48(m,1H,=CH),5.08-5.10(m,1H,=CH),4.87(d,2H,J=7.14Hz,CH 2 ),3.90(s,3H,ArOCH 3 ),2.10-2.14(m,4H,CH 2 CH 2 ),1.80(s,3H,CH 3 ),1.75(s,3H,CH 3 ),1.60(s,3H,CH 3 ).
[0038] The target compounds numbered I-2~I-4, I-12~I-14, I-18~I-25 and I-27~I-38 were obtained by the same method.
Embodiment 2
[0039] Example 2: Preparation of 3-hydroxysalicylic acid-(E)-3,7-dimethyl-2,6-octadienyl ester (I-5)
[0040] Step 1: Preparation of acetonitrile 3-hydroxysalicylate (intermediate 1)
[0041] In a 100ml three-neck flask, add 0.50g of 3-hydroxysalicylic acid, 0.29g of chloroacetonitrile, 0.49g of triethylamine and 20mL of acetone, and react under reflux for 6 hours. CH 2 Cl 2 Extract with water three times, take the organic phase, remove the solvent under reduced pressure, and separate by silica gel column chromatography to obtain acetonitrile 3-hydroxysalicylate as a yellow oily liquid with a yield of 75%.
[0042] Step 2: Preparation of 3-hydroxysalicylic acid-(E)-3,7-dimethyl-2,6-octadienyl ester (I-5)
[0043] In a 100ml three-necked flask, add 2.00g of acetonitrile 3-hydroxysalicylate, 2.19g of potassium carbonate, 1.80g of geraniol and 40mL of N,N-dimethylformamide, and react at 90°C for 6 hours. Extracted with water, removed the solvent under reduced pressure, and se...
Embodiment 3
[0045] Example 3: Preparation of 3-methyl-2-benzyloxy-salicylic acid-(E)-3,7-dimethyl-2,6-octadienyl ester (I-7)
[0046] Step 1: Preparation of benzyl 3-methyl-2-benzyloxysalicylate (intermediate 2)
[0047] In a 100ml three-neck flask, add 2.00g of 3-methylsalicylic acid, 6.75g of benzyl bromide, 1.59g of tri-NaOH, 0.68g of TBAB and 15mL of CH 2 Cl 2 , 15mL of water, and react at room temperature for 4 hours. Wash with water three times, take the organic phase, remove the solvent under reduced pressure, and separate by silica gel column chromatography to obtain light yellow liquid benzyl 3-methyl-2-benzyloxysalicylate with a yield of 97%.
[0048] Step 2: Preparation of 3-methyl-2-benzyloxysalicylic acid (intermediate 3)
[0049] In a 100ml three-necked flask, 3.18g of benzyl 3-methyl-2-benzyloxysalicylate, 1.10g of NaOH, and 35mL of ethanol were added, and the mixture was refluxed for 4 hours. The solvent was removed under reduced pressure and acidified with dilute hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com