Method for preparing high-purity doramectin
A doramectin, high-purity technology, applied in the field of doramectin preparation, can solve the problems of increased operation steps and production costs, low purity, environmental pollution, etc., to reduce process operations and equipment requirements, improve Product quality, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
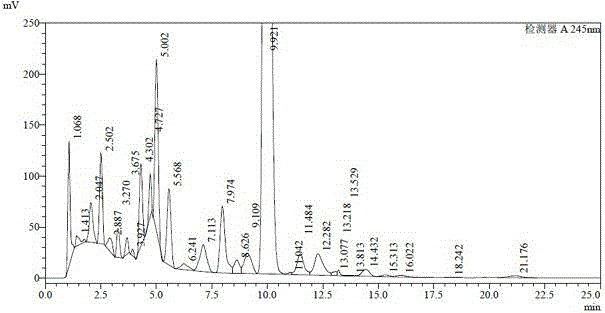
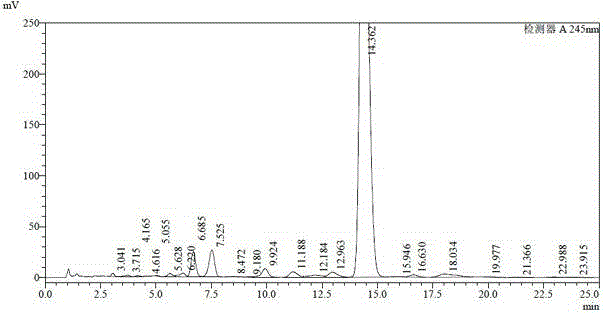
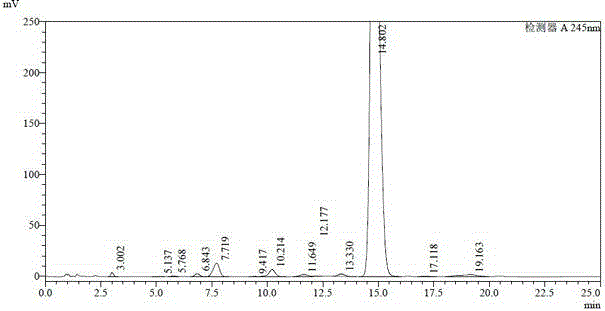
[0035] The doramectin fermentation broth was placed in a tank of 32.7L, and the fermentation unit was 1257ug / ml (41.1g). Add 490g of diatomaceous earth to it, stir for half an hour, perform plate and frame pressure filtration after uniformity, and obtain 4.2kg of wet mycelium residue, which is dried in a 45°C oven until the moisture content is 20%, and a 3.0kg block is obtained dry mycelium residue. Grind the mycelium residue into powder and extract with 9L butyl acetate, stir for 12 hours and filter to obtain about 9L extract (see HPLC spectrum figure 1 and its data are shown in Table 1), the unit is 3633ug / ml (32.7g). Concentrate the extract at 55°C until it is in the form of an extract, add a beating solution (methyl tert-butyl ether:ethanol=1.5:1 (v / v)) equal to the mass of the extract, stir at room temperature for 3 hours and then let it stand Overnight, filter to obtain light yellow crude product A (HPLC spectrum see figure 2 And its data are shown in Table 2) 87.3g...
Embodiment 2
[0048] Put the doramectin fermentation broth in a 35L tank, and the fermentation unit is 1000ug / ml (35g). Add 1.5 kg of diatomaceous earth therein, stir for half an hour, perform plate and frame pressure filtration after uniformity, and obtain 4.5 kg of wet mycelia residue. Grind the mycelial residue into powder and extract with 13.5L of ethanol, stir for 8 hours and then filter to obtain about 13.5L of extract with a unit of 2145ug / ml (29g). Concentrate the extract at 50°C until it is in the form of an extract, add a beating solution (methyl tert-butyl ether:ethanol=2:1 (v / v)) equal to the mass of the extract, stir at room temperature for 3 hours and then let it stand After overnight, it was filtered to obtain 78.6 g of pale yellow crude product A with a content of 36.5% (28.7 g) and a purity of 84.1%.
[0049] Add 700 ml of crystallization solution (isobutanol: acetone = 9:1 (v / v)) to the crude product A, stir at 50°C until the crude product A is just completely dissolved, ...
Embodiment 3
[0051] The doramectin fermentation broth was put into a tank of 31.2L, and the fermentation unit was 1100ug / ml (34.3g). Add 690g of diatomaceous earth to it, stir for half an hour, and perform plate and frame pressure filtration after uniformity to obtain 4.6kg of wet mycelium residue, which is dried in an oven at 45°C until the moisture content is 18%, and 3.2kg of block Dried mycelium residue, ground into powder, extracted with 13L of methanol, stirred for 15 hours and then filtered to obtain about 13L of extract, with a unit of 2110ug / ml (27.4g). Concentrate the extract at 55°C until it is in the form of an extract, add a beating solution (methyl tert-butyl ether:ethanol=1:1 (v / v)) equal to the mass of the extract, stir at room temperature for 3 hours and then let it stand After overnight, it was filtered to obtain 77.4 g of light yellow crude product A with a content of 32.8% (25.4 g) and a purity of 83.5%.
[0052] Add 470ml of crystallization solution (ethanol solution)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com