Disulfide bond isomerase gene Trpdi2 from Trichoderma reesei and application thereof
A technology of disulfide bond isomerase and Trichoderma reesei, which is applied in the field of biochemical engineering and microbial genetic engineering, can solve the problems of heavy load, lack of endoplasmic reticulum tool enzyme enzymes, protein misfolding and assembly, etc., to increase production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Cloning of Disulfide Bond Isomerase Gene
[0037] (1) Cloning of Trpdi2 Genomic DNA
[0038] According to the sequence information such as SEQ ID: 1 in the Trichoderma reesei data (http: / / genome.jgi-psf.org / Trire2 / Trire2.home.html), design relevant primers, the primer sequence is:
[0039] Trpdi2-F: ATGGTCTTGATCAAGAGCCTC,
[0040] Trpdi2-R: TCACAGCTCGTCCTTCTGG,
[0041] Using the genomic DNA of the QM9414 strain stored at -20°C as a template, PCR amplification was performed to obtain the full-length DNA fragment of the gene, which was cloned into the pGEM-T vector.
[0042] Wherein, the PCR reaction system is:
[0043]
[0044]
[0045] The PCR reaction conditions are:
[0046]
[0047] (2) Cloning of Trpdi2 cDNA
[0048] Using the cDNA of QM9414 strain stored at -80°C as a template, the cDNA fragment was amplified by PCR with primers Trpdi2-F and Trpdi2-R, and cloned into pGEM-T vector.
[0049] in,
[0050] The PCR reaction system is:
[00...
Embodiment 2
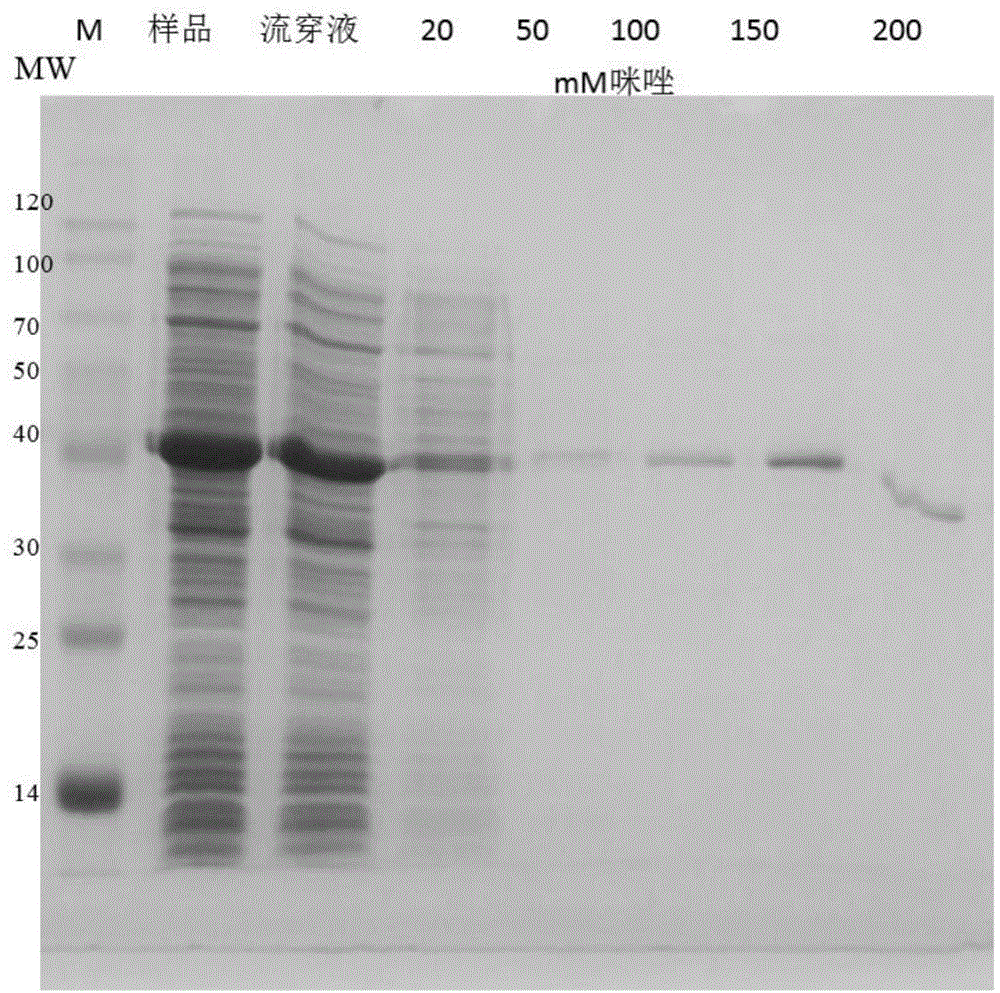
[0054] Example 2: Expression and Activity Measurement of Disulfide Bond Isomerase
[0055] (1) Expression vector construction of disulfide bond isomerase gene Trpdi2
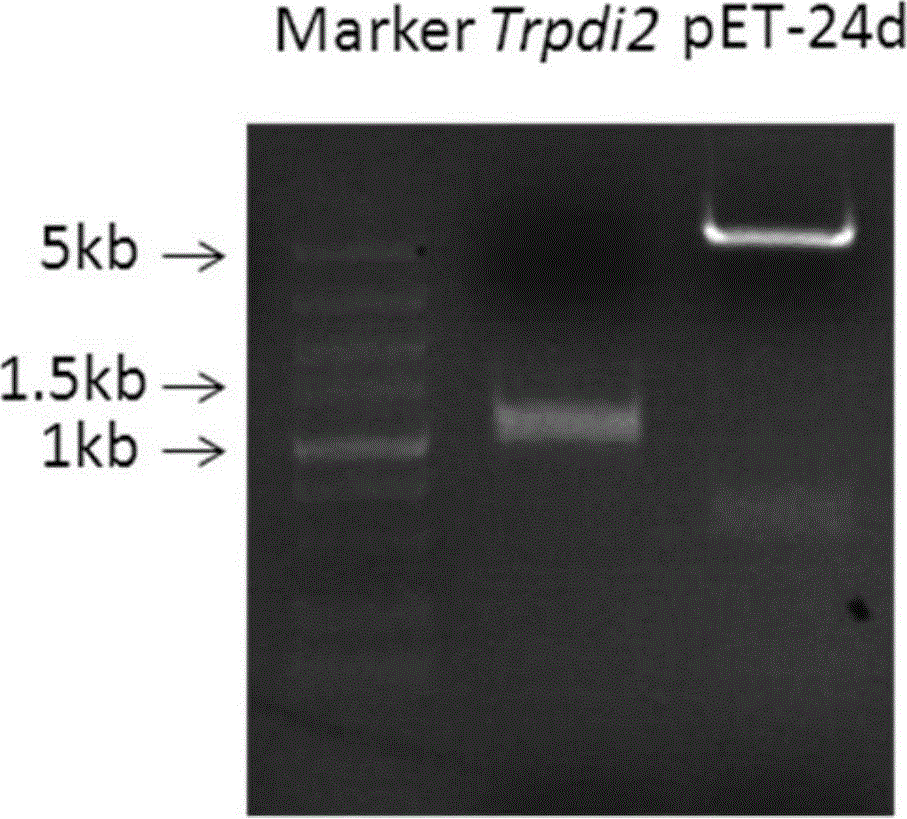
[0056] According to the sequence of the disulfide bond isomerase gene Trpdi2, primers with restriction sites at the end were designed, and the cDNA of the QM9414 strain stored at -80°C was used as a template to perform PCR amplification to obtain the cDNA containing the sequence of the disulfide bond isomerase gene Trpdi2 DNA fragments, DNA fragments recovered by NcoI and BamHI double enzyme digestion and the pET-24d vector preserved in our laboratory, such as figure 1 shown. The two restriction fragments were recovered and ligated to obtain an expression vector, and the obtained expression vector was transformed into Escherichia coli DH5α. The transformed Escherichia coli DH5α was sequenced and detected, and the sequenced detection proved that the transformed DH5α was correct, and the extracted plasmid was tr...
Embodiment 3
[0065] Example 3: Effect of Disulfide Bond Isomerase on Exoglucanase Activity
[0066] (1) Effect of disulfide bond on the activity of exoglucanase CBH1 protein
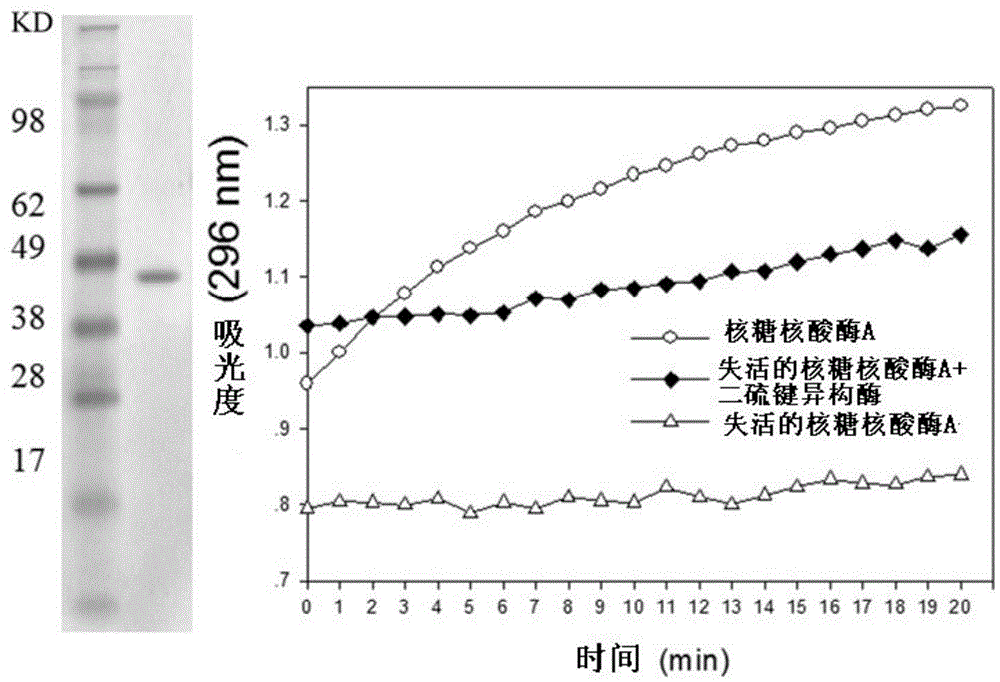
[0067] Using Trichoderma reesei fermentation liquid as a sample, CBH1 pure protein was separated and purified by DEAE protein chromatography column. CBH1 was treated with different concentrations of DTT, and the activity of CBH1 protein was measured after 1, 2, 3, 4, 5, 6 and 18 hours of treatment. The activity was determined based on the activity of the substrate pNPC. Such as Figure 4 As shown, the left side is the SDS polyacrylamide gel electrophoresis image of the purified CBH1 protein sample, and the right side is the result graph of CBH1 activity after DTT treatment for different time, in which three curves represent the pNPC enzyme activity of CBH1 after different time treatment, With the treatment time of DTT, the activity of CBH1 enzyme decreased; with the increase of DTT treatment concentration, the acti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com