a ws 2 Method of inhibiting Aβ aggregation by nanosheets and method of deaggregating formed Aβ fiber aggregates
A technology of nanosheets and aggregates, applied in the field of medicine, can solve problems such as low blood-brain barrier permeability, high toxic and side effects, and weak depolymerization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] WS 2 Nanosheets inhibit the self-aggregation of Aβ1-40:
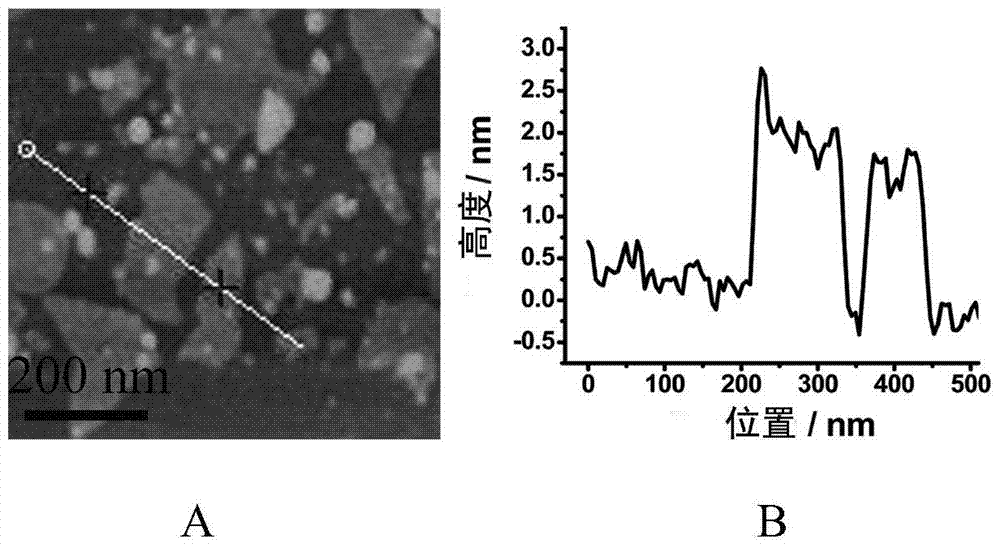
[0047] Step 1: Prepare WS 2 Nanosheet: 100mg of WS in a nitrogen environment 2 The powder was dissolved in 1.6 M n-butyllithium solution and reacted. After 2 days, the mixture was filtered with Whatman#41 filter paper and washed with 100 mL of hexane 3 times. 300 mL of deionized water was added to the semi-dry precipitate obtained, and ultrasonic treatment 1 After hours, the solution was centrifuged, washed with deionized water 3 times to remove lithium ions and undispersed large particles, the supernatant was collected, and dialyzed with deionized water for 5 days to obtain WS 2 Nanosheets;
[0048] Step 2: First, dissolve Aβ1-40 (product number U10012, purchased from American Peptide) in hexafluoroisopropanol at a concentration of 1mg / ml, seal the bottle, and place it at 4℃ for 2 hours to shake It is completely dissolved and the stock solution is stored at -20°C. Before use, dry the hexafluoroisopropanol with a gent...
Embodiment 2
[0054] WS 2 Aβ1-40 fiber aggregates formed by photothermal depolymerization of nanosheets:
[0055] Step 1: Prepare WS 2 Nanosheet: 100mg of WS in a nitrogen environment 2 The powder was dissolved in 1.6 M n-butyllithium solution and reacted. After 2 days, the mixture was filtered with Whatman#41 filter paper and washed with 100 mL of hexane 3 times. 300 mL of deionized water was added to the semi-dry precipitate obtained, and ultrasonic treatment 1 After hours, the solution was centrifuged, washed with deionized water 3 times to remove lithium ions and undispersed large particles, the supernatant was collected, and dialyzed with deionized water for 5 days to obtain WS 2 Nanosheets;
[0056] Step 2: First, dissolve Aβ1-40 (product number U10012, purchased from American Peptide) in hexafluoroisopropanol at a concentration of 1mg / ml, seal the bottle, and place it at 4℃ for 2 hours to shake It is completely dissolved and the stock solution is stored at -20°C. Before use, dry the hexaf...
Embodiment 3
[0060] WS 2 Nanosheets inhibit Aβ1-40 aggregation in cerebrospinal fluid
[0061] The reaction conditions and steps are the same as those in Example 1, except that the treated Aβ1-40 and WS 2 The nanosheets are incubated in the cerebrospinal fluid.
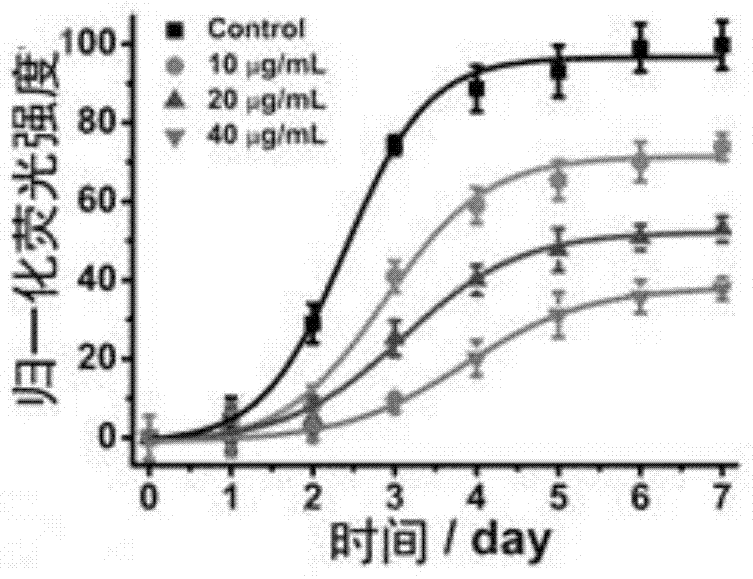
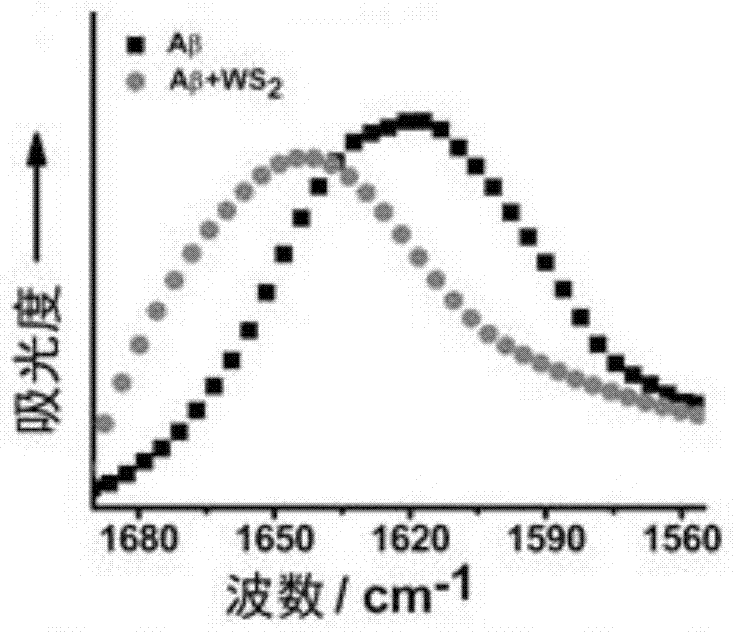
[0062] Picture 9 Example 3WS 2 The ThT fluorescence detection chart of the inhibitory effect of nanosheets on the aggregation of Aβ1-40, Figure 9 shows that in the cerebrospinal fluid, WS 2 Nanoflakes can still inhibit the Aβ1-40 fibrosis process in a concentration-dependent manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com