Method for removing endotoxin of biological product
A technology for biological products and endotoxins, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor removal effect and high cost, and achieve clinical application prospects, low cost, and treatment methods simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 endotoxin removal method of the present invention
[0031] 1. Test material
[0032] Antibody protein sample acquisition:
[0033] Step 1. Preparation of a CHO cell capable of expressing an anti-CD20 monoclonal antibody:
[0034] Preparation of CHO cells expressing anti-CD20 monoclonal antibody: The dhfr (dihydrofolate reductase) expression unit in the pSV2-dhfr vector (ATCC product) was cloned into the pCDNA3.1 (+) vector (Invitrogen Company product), the mammalian cell expression vector pBF01 capable of expressing DHFR was constructed.
[0035] According to literature reports (US Patent, US6399061), the light chain and heavy chain gene fragments of the recombinant anti-CD20 monoclonal antibody were synthesized by chemical synthesis technology; the synthetic gene fragments were respectively cloned into pBF01 vector by DNA recombination technology, and express The recombinant expression vectors pBF01-CD20L and pBF01-CD20H of the light chain and heavy cha...
Embodiment 2
[0070] Embodiment 2 endotoxin removal method of the present invention
[0071] 1. Test material
[0072] With embodiment 1.
[0073] 2. Endotoxin removal method of the present invention
[0074] Except for the parameters changed in the following Tables 1 to 2, other conditions are the same as in Example 1.
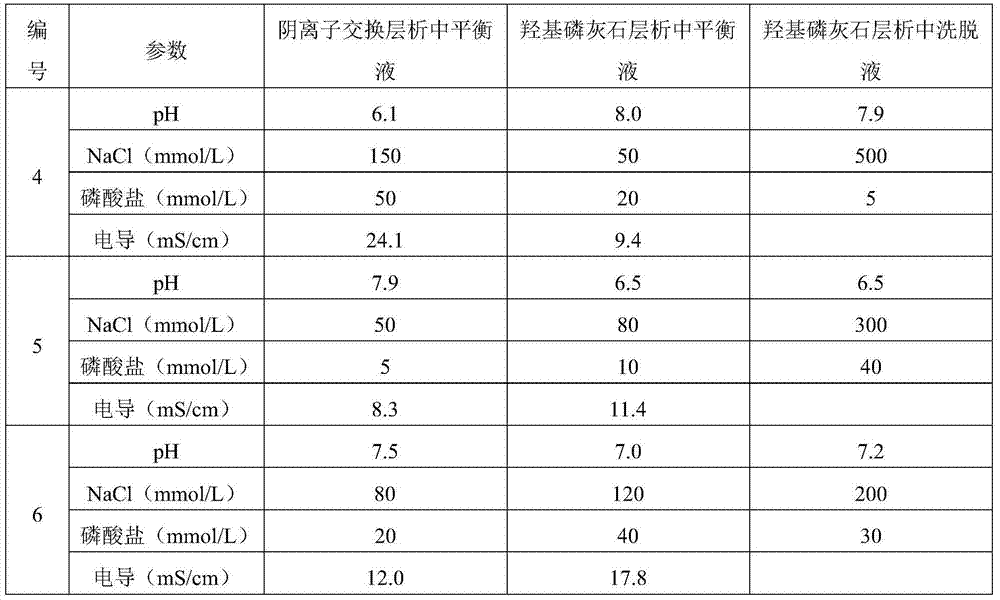
[0075] Table 1 Changes of anion resin type and hydroxyapatite type
[0076] Numbering
Anion resin type
Hydroxyapatite type
1
Q Sepharose High Performance
CHT Ceramic hydroxyapatite type I,40um
2
CHT Ceramic hydroxyapatite type II,40um
3
Nuvia Q
CHT Ceramic hydroxyapatite type I,80um
[0077] Table 2 Changes in the buffer system
[0078]
[0079] 3. Detection and analysis
[0080] With embodiment 1.
[0081] 4. Experimental results
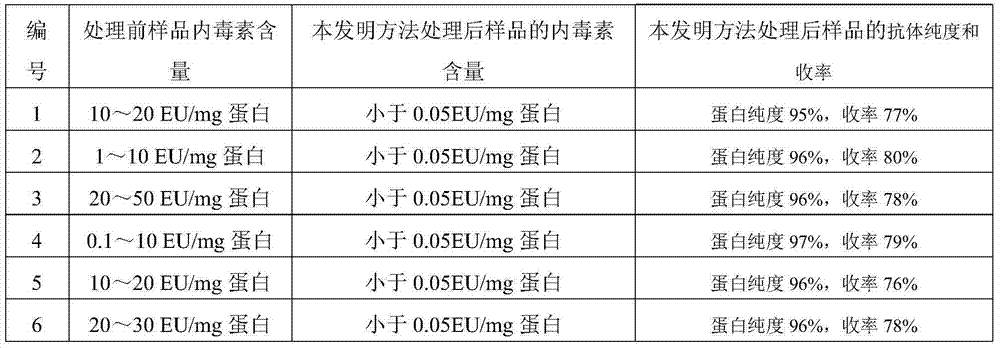
[0082] Table 3 Test results
[0083]
[0084] As can be seen from Table 3, before adopting the method of the present invention to proce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com