Oxytetracycline preparation and preparation method
A technology for oxytetracycline preparations, which is applied in the field of oxytetracycline preparations and preparations, can solve the problems of drug unevenness, easy precipitation, and influence on drug efficacy, and achieve the effects of shortening production time, high production efficiency, and increasing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
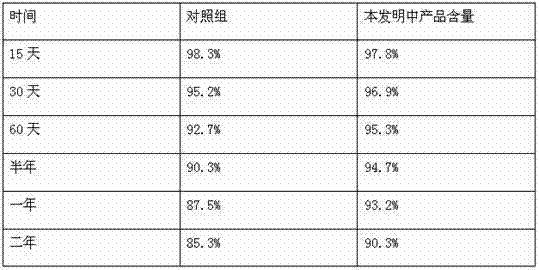
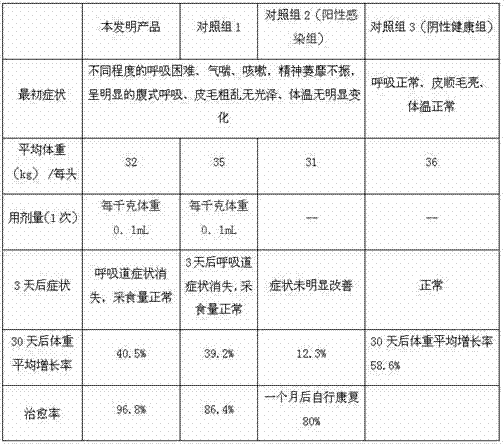
[0023] Magnesium chloride is ultrafinely pulverized, and after pulverization, the pulverization fineness is ≥500 mesh; take part of the water for injection, add 0.5% sodium formaldehyde sulfoxylate and stir to dissolve, and then add 45% of α-pyrrolidone, 30% of oxytetracycline and 2% of magnesium chloride Form a mixed solution, then heat the mixed solution to 60°C, stir continuously, and keep it warm for 35 minutes; use the remaining water for injection to make up the volume, stir evenly, and adjust the pH to 8-9 with 2-5% ethanolamine.
[0024] Water for injection, that is, the water obtained by distilling purified water. Its pH value is 5; ammonia does not exceed 0.0002%; nitrate does not exceed 0.000006%; nitrite does not exceed 0.000002%; conductivity: when 10ml or less, the limit is 25μS / cm; The amount should be less than 0.25EU; microbial limit: no more than 10 bacteria and yeast per 100ml.
Embodiment 2
[0026] Magnesium chloride is ultrafinely pulverized, and after pulverization, the pulverization fineness is ≥500 mesh; take part of the water for injection, add 2% formaldehyde and sodium bisulfite, stir and dissolve, and then add 55% of α-pyrrolidone, 30% of oxytetracycline and 3% of magnesium chloride Form a mixed solution, then heat the mixed solution to 70°C, stir continuously, and keep warm for 25 minutes; use the remaining water for injection to make up the volume, stir evenly, and adjust the pH to 8-9 with 2-5% ethanolamine.
[0027] Water for injection, that is, the water obtained by distilling purified water. Its pH value is 6; ammonia does not exceed 0.0002%; nitrate does not exceed 0.000006%; nitrite does not exceed 0.000002%; conductivity: 10ml or less, the limit is 25μS / cm; The amount should be less than 0.25EU; microbial limit: no more than 10 bacteria and yeast per 100ml.
Embodiment 3
[0029] Superfinely pulverize 2-3% magnesium chloride, after pulverization, the pulverization fineness is ≥500 mesh; take part of the water for injection, add 1.5% sodium formaldehyde sulfoxylate and stir to dissolve, then add α-pyrrolidone 46%, oxytetracycline 30% Make a mixed solution with 2.2% magnesium chloride, then heat the mixed solution to 65°C, keep stirring, and keep it warm for 35 minutes; use the remaining water for injection to make up the volume, stir evenly, and adjust the pH to 8-9 with 2.5% ethanolamine.
[0030] Water for injection, that is, the water obtained by distilling purified water. Its pH value is 7; ammonia does not exceed 0.0002%; nitrate does not exceed 0.000006%; nitrite does not exceed 0.000002%; conductivity: when 10ml or less, the limit is 25μS / cm; The amount should be less than 0.25EU; microbial limit: no more than 10 bacteria and yeast per 100ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grinding fineness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com